- Title
-
Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord
- Authors
- Kida, Y.S., Sato, T., Miyasaka, K.Y., Suto, A., and Ogura, T.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
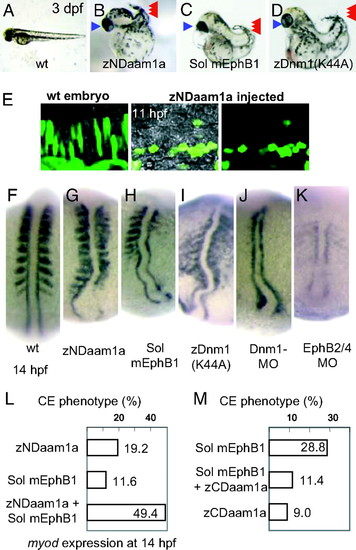
Disruption of the noncanonical Wnt pathway or the EphB signaling cascade induces the same CE phenotype. (A–D) Morphology [72 hours postfertilization (hpf)] of the wild-type embryo (A), embryo injected with zebrafish NDaam1a mRNA (B), mRNA encoding a soluble form of mouse EphB1 (C), and mRNA encoding a K44A mutant of zebrafish Dnm1 (D). These embryos share a short and bent body axis (red arrowheads), as well as cyclopia (blue arrowheads). (E) EGFP protein was expressed in the notochord cells to visualize their shape. In the wild-type embryo, the notochord cells elongate to show the intercalation movement. When zNDaam1 was expressed, the notochord cells became round and did not develop a polarized shape. Dorsal views at 11 hpf are shown. Rostral to the left, caudal to the right. (F) At 14 hpf, myoD is expressed in the somites and the adaxial cells in the wild type. (G) Injection of zNDaam1 mRNA resulted in a short body axis with compressed somites. (H and I) Injection of mRNA encoding the soluble form of mouse EphB1 (H) or the dominant-negative dynamin1 [Dnm1(K44A)] (I) induced a similar phenotype. (J) Injection of Dnm1-MO resulted in similar but more severe defects. (K) Coinjection of EphB4-MO and EphB2-MO induced severe defects. The notochord was short, broader, and bent. Pictures shown in F–K are shown at the same magnification. (L) Injection of mRNA encoding zNDaam1 or the soluble form of EphB1 induced the abnormalities of CE movement in 19.2% or 11.6% of injected embryos, respectively, as judged by expression of myoD at 14 hpf. Coinjection of these mRNAs induced the same abnormality in 49.4% of embryos. (M) Injection of a soluble mEphB1-FC mRNA induced the CE defect in 29.8% of injected embryos. Coinjection with zCDaam1a mRNA rescued this defect, and the CE abnormality was observed in only 11.4% of embryos. Injection of zCDaam1 mRNA alone induced the defect in 9.0% of embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Subcellular localization of Daam1 in notochord cells. (A) Serial pictures of a notochord cell taken at 20-min intervals. EGFP-Daam1-positive vesicles (red arrows) moved from the caudal cell surface to the center (green arrowhead). Daam1-positive fibers were formed on the caudal side of the cell (blue line). (B) Serial pictures of another notochord cell taken at 4-min intervals. EGFP-Daam1-positive vesicles (red arrows) moved from the caudal cell surface to the center (green arrowhead). (C) Serial pictures of the cell shown in (A). GFP-tagged Daam1 accumulated in the fibers near the caudal cell surface (blue bars). (D and E) Subcellular localization of GFP-tagged Daam1 is shown in green. At this stage, Daam1 colocalized with F-actin in the cell cortex and the fibers formed near the caudal surface (yellow arrowheads). Note that the colocalization is more evident at the lateral ends that make tight junctions with surrounding tissues (blue arrowheads in E). (F) When wnt11 MO was injected, the notochord cells did not elongate but, rather, formed small spiky protrusions where Daam1 colocalized. Note that Daam1 did not localize in the endocytic vesicles or the fibers in the cell cortex and, instead, stayed at the cell membrane. PHENOTYPE:
|
|
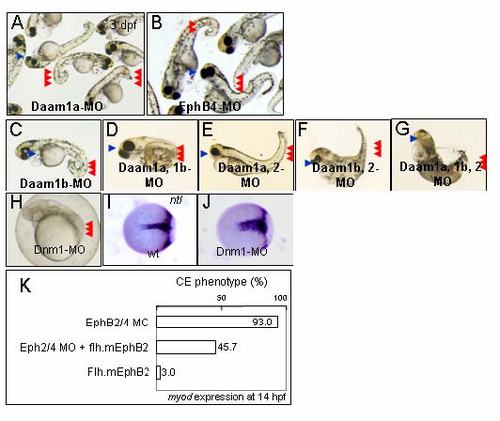
Phenotypes of Daam morphants. (A) Injection of a morpholino oligonucleotide (MO) against Daam1a induced a short bent tail (red arrowheads) and cyclopia (blue arrowhead) at 3 d after fertilization (dpf). (B) Injection of a MO against EphB4 resulted in similar defects with a short bent tail (red arrowheads) and cyclopia (blue arrowhead). (C) Injection of a Daam1b MO also induced a similar phenotype. (D) Coinjection of Daam1a- and Daam1b MOs resulted in more severe abnormalities, consisting of a short bent tail (red arrowheads) and cyclopia (blue arrowhead). (E) In contrast, phenotype induced by coinjection of Daam1a- and Daam2 MOs was similar to that induced by the Daam1a-MO single injection (A). (F) Likewise, coinjection of Daam1b- and Daam2 MOs resulted in the same defect as the Daam1b MO (C). These observations indicate that Daam1a and Daam1b function in a redundant manner. (G) Triple injection of Daam1a-, Daam1b-, and Daam2 MOs induced more severe effects, with loss of eyes (blue arrowhead) and a short bent tail (red arrowheads). Because injection of Daam2 MO alone did not induce any obvious alteration, the contribution of Daam2 to notochord development is small (data not shown). (H) Loss-of-function of Dynamin1 (Dnm1) results in the phenotype characteristic of abnormal CE cell movement, consisting of compressed somites (red arrowheads) and a short body axis. (I and J) At the 80% epiboly stage, distribution of mesoderm cells marked by ntl became wider and shorter (J) than the wild-type embryo (I). (K) Coinjection of MOs against EphB2 and EphB4 induced the CE defects in 93.0% of embryos (n = 44). Coinjection of an flh-mEphB2 plasmid, which drives expression of mouse EphB2 in the notochord, rescued the defects, which were observed in 45.7% of injected embryos (n = 35). Injection of flh-mEphB2 alone induced the defects in only 3.0% of embryos (n = 33). EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|