- Title
-
Amotl2 is essential for cell movements in zebrafish embryo and regulates c-Src translocation
- Authors
- Huang, H., Lu, F.I., Jia, S., Meng, S., Cao, Y., Wang, Y., Ma, W., Yin, K., Wen, Z., Peng, J., Thisse, C., Thisse, B., and Meng, A.
- Source
- Full text @ Development
|
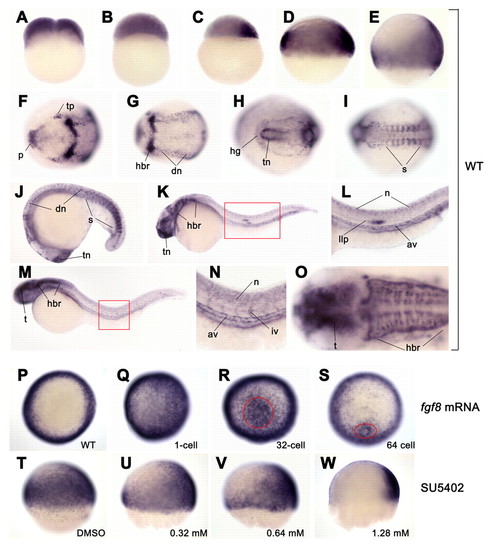
amotl2 expression and regulation by Fgf signal in zebrafish embryos. (A-O) amotl2 expression in wild type was detected by whole-mount in situ hybridization at two-cell (A), 1k-cell (B), sphere (C), shield (D), 70% epiboly (E), two-somite (F,G), ten-somite (H,I), 18-somite (J), 24 hpf (K,I) and 36 hpf (M-O) stages. (A-E) Lateral views with dorsal to the right; (F-I,O) dorsal views with anterior to the left; (J-N) lateral views with anterior to the left. (F and G; H and I) The anterior (F,H) and posterior (G,I) regions for the same embryo, respectively. (L) Higher magnification of the region boxed in K. (N) Higher magnification of the region boxed in M. (O) Head of the embryo in M. (P-W) amotl2 expression in embryos injected with 14 pg fgf8 mRNA (Q-S) or treated with Fgfr signaling inhibitor SU5402 (U-W). (P-S) Animal pole views at the shield stage with dorsal to the right. (T-W) Lateral views at the 60% epiboly stage with dorsal to the right. fgf8 mRNA was injected into a one-cell embryo (Q) or a single cell located in the animal pole at the 32-cell stage (R) or the 64-cell stage (S). amotl2 expression was strongly induced in the circled area following single-cell injection at multi-cell stages (R,S). SU5402 treatment started at the sphere stage. av, axial vasculature; dn, dorsal neuron; hbr, hindbrain rhombomere; hg: hatching gland; iv, intersegmental vessel; llp, lateral line primordium; n, neuron; p, polster; s, somite; t, tectum; tn, telencephalon; tp, trigeminal placodes; vn, ventral neuron. |
|
Effectiveness of amotl2 morphants. Embryos injected with 50 pg amotl2-GFP plasmid DNA showed green fluorescence at the shield stage (A). When co-injected with amotl2MO1 (MO1) or amotl2MO2 (MO2), green fluorescence decreased in a dose-dependent way (B-D,F-H). However, co-injection with cMO1 did not inhibit production of Amotl2-GFP fusion protein (E). Note that a higher dose of MO2 was required to inhibit Amotl2-GFP expression. |
|
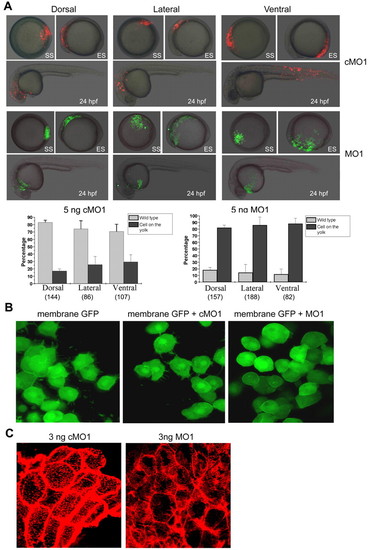
Effects of amotl2 expression knockdown on embryonic cell movements. (A) Injection of amotl2 morpholino led to slower epiboly and impaired CE movement. Single-cell embryos were injected with amotl2MO1 (MO1), amotl2MO2 (MO2) or control morpholino (cMO1) at the indicated doses. The same embryo was pictured every 2 hours and presented here. Embryos are shown as lateral views with animal pole to the top and dorsal to the right. Arrows denote the anterior tip of the hypoblast, and arrowheads point to the tail region. (B) Injection of amotl2 morpholino affected convergent extension. Injected embryos at the two-somite stage were fixed and simultaneously hybridized to hgg1 (red), dlx3 (blue) and ntl (blue) probes. Each embryo was placed in different orientation: in the top panel, anterodorsal views show relative positions of the hgg1 and dlx3 domains; in the middle panel, dorsal views show the ntl domain; and in the bottom panel, lateral views with dorsal to the right show the length of the ntl domain and the embryonic anteroposterior axis. (C) Effect of amotl2 morpholino injection was alleviated by overexpression of modified amotl2 (amotl2m) mRNA. Groups of live embryos were pictured at the indicated stages. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Effects of amotl2 expression knockdown on embryonic cell movements. (A) Injection of amotl2 morpholino led to slower epiboly and impaired CE movement. Single-cell embryos were injected with amotl2MO1 (MO1), amotl2MO2 (MO2) or control morpholino (cMO1) at the indicated doses. The same embryo was pictured every 2 hours and presented here. Embryos are shown as lateral views with animal pole to the top and dorsal to the right. Arrows denote the anterior tip of the hypoblast, and arrowheads point to the tail region. (B) Injection of amotl2 morpholino affected convergent extension. Injected embryos at the two-somite stage were fixed and simultaneously hybridized to hgg1 (red), dlx3 (blue) and ntl (blue) probes. Each embryo was placed in different orientation: in the top panel, anterodorsal views show relative positions of the hgg1 and dlx3 domains; in the middle panel, dorsal views show the ntl domain; and in the bottom panel, lateral views with dorsal to the right show the length of the ntl domain and the embryonic anteroposterior axis. (C) Effect of amotl2 morpholino injection was alleviated by overexpression of modified amotl2 (amotl2m) mRNA. Groups of live embryos were pictured at the indicated stages. PHENOTYPE:
|
|
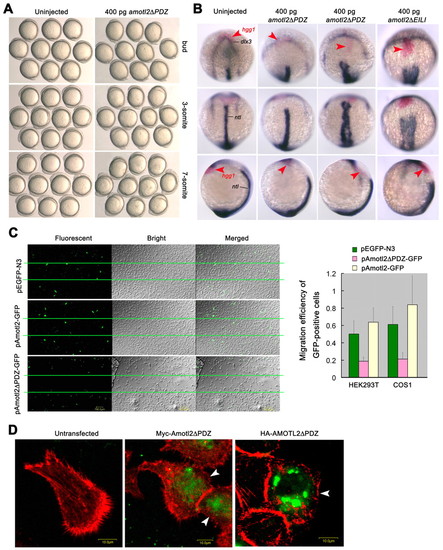
Effect of amotl2 mutants on cell migration and structures. (A) Effect of overexpression of amotl2ΔPDZ mutant on cell movements of zebrafish embryos. Embryos were injected at the one-cell stage and observed at the indicated stages. Embryos were managed to position with animal pole or anterior to the top. (B) Expression of hgg1 (red), dlx3 (blue) and ntl (blue) at the two-somite stage. Top panel, anterodorsal views; middle panel, dorsal views with anterior to the top; bottom panel, lateral views with anterior to the top and dorsal to the right. Injection of amotl2ΔPDZ mRNA led to varying degrees of defects in convergent extension (in the second and third columns). Injection of amotl2ΔEILI mRNA also caused defective convergent extension (fourth column). The hgg1 expression domain is indicated by arrowheads. (C) Expression of Amotl2ΔPDZ-GFP or Amotl2-GFP inhibited or promoted cell migration in vitro, respectively. Wound-healing assays were done both in HEK293T (shown on the left) and in COS1 cells. The wound area was between two lines. The bar graph at the right shows statistical data from three experiments with standard deviations. The migration efficiency of GFP-positive cells was calculated as percentage of GFP-positive cells in the wound-healing area/percentage of GFP-positive cells in the non-wound area. (D) F-actin distribution was abnormal in HeLa cells transfected with either Myc-Amotl2ΔPDZ (derived from fish) or HA-AMOTL2ΔPDZ (derived from human). Cells were stained with phalloidin 24 hours after transfection. Arrowheads indicate Amotl2-expressing cells. Scale bars: 10 μm. EXPRESSION / LABELING:
|
|
Amotl2 associates with and promotes peripheral translocation of c-Src. (A) HA-Amotl2 was coexpressed with Src-WT-GFP or Src-Y527F-GFP in HEK293T cells and their interaction was examined by reciprocal immunoprecipitation. (B) Overexpressed HA-Amotl2 associated with endogenous c-SRC in human HEK293T cells. HA-Amotl2 was immunoprecipitated with anti-HA antibody and the precipitates were examined by western blotting for the presence of c-Src (indicated by an arrow) with anti-c-Src antibody. TCL, total cell lysate. (C) Overexpressed HA-Amotl2 or Myc-Amotl2 was co-localized with overexpressed Src-WT-GFP (upper panel) or endogenous c-Src (lower panel) in HeLa cells. (D) Examination of Amotl2 association with related tyrosine kinases Fyn and Yes of zebrafish. Myc-Amotl2 was coexpressed with HA-Src, HA-Fyn or HA-Yes in human HEK293T cells. Reciprocal co-immunoprecipitation was performed. Note that Myc-Amotl2 was co-precipitated with HA-Src (indicated by arrows), but not with HA-Fyn or HA-Yes. (E) Effect of Amotl2 on translocation of exogenous active c-Src. HeLa cells were transfected with Src-Y527F-GFP alone, or in combination with Myc-Amotl2 or Myc-Amotl2ΔPDZ, and treated with bradykinin. Lower panel: high magnification of an area boxed in the corresponding cell in the upper panel. (F) Effect of Amotl2 on translocation of endogenous phosphorylated c-Src in HeLa cells. Following transfection with Myc-Amotl2 (upper panel) or Myc-Amotl2ΔPDZ (lower panel), cells were treated with bradykinin and stained with p-c-Src (Thr420) antibody. (G) Co-localization of Myc-Amotl2 with endogenous endosomal proteins RhoB (upper panel) and EEA1 (lower panel) in HeLa cells. Scale bars: 10 μm. |

Unillustrated author statements PHENOTYPE:
|