- Title
-
STAT3-dependent pathfinding and control of axonal branching and target selection
- Authors
- Conway, G.
- Source
- Full text @ Dev. Biol.
|
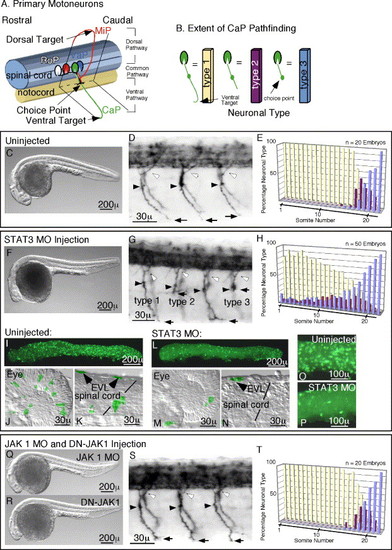
Morpholino (MO) Inactivation of STAT3, but not JAK1, causes Pathfinding defects. In panels D, G, and S, white arrowheads mark dorsal projections, black arrowheads the choice point, black arrows mark the most ventral extension of the CaP motoneuron axon. (A) Schematic diagram of primary motoneurons in the zebrafish showing motoneuron subtypes: RoP, MiP, CaP, and VaP, and their axon projections. (B) Schematic diagram of CaP neural types based upon extent of axon pathfinding used in quantitations shown in panels E, H, G, T, and in Fig. 2 and Fig. 3. (C–E) Analysis of uninjected embryos. (C) Whole body image of an uninjected embryo showing the normal body axis. (D) Normal primary motoneuron projections in an uninjected embryo. (E) Quantitation of CaP neural types in uninjected embryos, showing type 2 and 3 neurons confined to caudal somites. (F–H) Analysis of STAT3 MO injected embryos. (F) Whole body image of a STAT3 MO injected embryo showing foreshortened anterior axis with subsequent fore and mid-brain defects. These defects result from reduced anterior axial mesendoderm cell migration, a morphotype of STAT3 inactivation. (G) Primary motoneuron projections in a STAT3 MO injected embryo showing forestalled ventral CaP axon projections. (H) Quantitation of CaP neural types in STAT3 MO embryos showing stalled type 2 and 3 neurons in rostral as well as caudal somites. (I–P) Comparison of activated STAT3 in uninjected and STAT3 MO injected embryos. (I and L) Whole embryo images of phospho-STAT3 containing cells in uninjected (I) and STAT3 MO injected (L) embryos. (J and M) Images of the eye showing phospho-STAT3-positive cells in uninjected (J) and STAT3 MO injected (M) embryos. (K and N) Images of the spinal cord showing the presence of phospho-STAT3 containing neurons in uninjected (K) and STAT3 MO injected (N) embryos. (O and P) Close up of EVL cells expressing activated STAT3 in an uninjected embryo (O) and reduced cell numbers in a STAT3 MO injected embryo (P). (Q–T) Analysis of JAK1 MO injected and DN-JAK1 expressing embryos. (Q) Whole body image of a JAK1 MO injected embryo showing foreshortened anterior axis with subsequent fore and mid-brain defects. These defects result from reduced anterior axial mesendoderm cell migration, a morphotype indicative of JAK1 inactivation. (R) Whole body image of a DN-JAK1 expressing embryo showing anterior axis defects identical to that see in panel Q above. (S) Primary motoneuron projections in a JAK1 MO injected embryo, showing normal axonal trajectories. Pathfinding is also unaffected in DN-JAK1 expressing embryos. (T) Quantitation of CaP neural types in JAK1 MO injected embryos, showing type 2 and 3 neurons confined to caudal somites as is seen for uninjected embryos. Identical results were obtained for DN-JAK1 expressing embryos. In all images, rostral is to the left, and dorsal is up. EXPRESSION / LABELING:
|
|
Blocking peptides result in pathfinding failures. In panels B, E, and H, black arrowheads mark the choice point and black arrows mark the most ventral extension of the CaP motoneuron axon. (A–C) Analysis of control peptide injected embryos. (A) Whole body image of an embryo injected with the control peptide showing the normal body axis. (B) Normal primary motoneuron projections. (C) Quantitation of CaP neural types in control peptide injected embryos, showing type 2 and 3 neurons confined to caudal somites as in uninjected embryos. (D–F) Analysis of embryos injected with phosphotyrosine containing peptide. (D) Whole body image of an embryo injected with the phosphotyrosine containing peptide showing foreshortened anterior axis with subsequent fore and mid-brain defects. (E) Primary motoneuron projections showing forestalled ventral CaP axon projections. (F) Quantitation of CaP neural types showing stalled type 2 and 3 neurons in rostral as well as caudal somites. (G–I) Analysis of embryos injected with phosphomethyl-Phe containing peptide. (G) Whole body image showing foreshortened anterior axis with subsequent fore and mid-brain defects. (H) Primary motoneuron projections showing forestalled ventral CaP axon projections. (I) Quantitation of CaP neural types showing stalled type 2 and 3 neurons in rostral as well as caudal somites. In all images, rostral is to the left, and dorsal is up. |
|
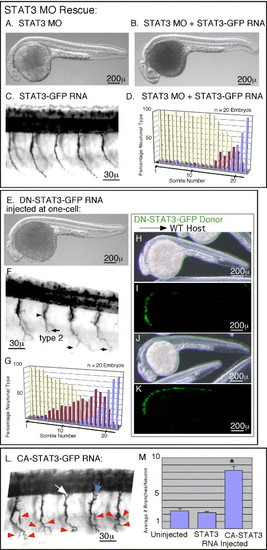
Morpholino defects can be rescued, while Dominant negative and Constitutively Active versions differentially alter Pathfinding. In panel F, black arrowheads mark the choice point and black arrows mark the most ventral extension of the CaP motoneuron axon. In panel L, red arrowheads mark axon branches. White and blue arrows in panel L are mentioned in the Results section on mosaic analysis. (A–D) Rescue of Morpholino induced pathfinding defects. (A) Whole body image showing foreshortened anterior axis with subsequent fore and mid-brain defects. (B) Whole body image showing rescue of the anterior axis by co-injection of RNA encoding wild-type STAT3 with the STAT3 MO. (C) Primary motoneuron projections in an embryo injected with RNA encoding wild-type STAT3, showing no affect upon pathfinding. (D) Quantitation of CaP neural types showing rescue of pathfinding defects by co-injection of MO with RNA encoding wild-type STAT3. (E–G) Analysis of embryos expressing DN-STAT3. (E) Whole body image showing foreshortened anterior axis with subsequent fore and mid-brain defects. (F) Primary motoneuron projections showing forestalled ventral CaP axon projections. (G) Quantitation of CaP neural types showing stalled type 2 and 3 neurons in rostral as well as caudal somites. (H–K) Confirmation that DN-STAT3 inhibits endogenous signaling by demonstration of nonautonomous cell migration defects. (H) Whole body image of a wild-type embryo showing foreshortened anterior axis with subsequent fore and mid-brain defects due to transplantation of DN-STAT3 expressing cells populating anterior axial mesendoderm. Because these cells contribute to precordal mesendoderm, they inhibited migration of neighboring cells along the anterior axis. This noncell autonomous affect during gastrulation is a characteristic of STAT3 signaling. (I) GFP fluorescence of donor cells populating anterior axial mesendoderm of embryo shown in panel H. (J) Whole body image of a wild-type embryo showing normal anterior axis because DN-STAT3 expressing cells did not populate the precordal plate. (K) The donor cells, shown by GFP fluorescence, positioned more posterior to those in panels H and I, and thus not inhibiting migration of neighboring blastomeres. (L and M) Constitutive activation of STAT3 leads to exuberant branching. (L) Primary motoneuron projections showing extra branches on the CaP motoneuron axon. (M) Quantitation of branch numbers. Asterisk marks statistical significance (t test, P < 0.05). In all images, rostral is to the left, and dorsal is up. Embryos are at 27 h post-fertilization. |
|
Mosaic analysis reveals cell autonomous requirements for, and affects of, STAT3 activation upon CaP motoneuron pathfinding. White arrowheads in all panels mark donor cells that did not differentiate into neurons; most are EVL cells. Panels A–L show embryos at 27 h post-fertilization (p.f.). Embryos in panels M–P are at 48 h p.f. In all images, rostral is to the left, and dorsal is up. (A–L) Cell transplantation chimeras show a cell autonomous requirement for STAT3 in CaP motoneuron pathfinding and axon bifurcation upon constitutive activation. (A, C, E, G, I, K, and L) Donor motoneurons transplanted into host embryos revealing axon projections of the donor neurons. (B, D, F, H, and J) ZNP1 staining of primary motoneurons present in the preceding panel. (B) Wild-type donor CaP transplanted into a wild-type host showing that the transplant technique does not alter axon projections or donor viability. (C) Wild-type donor CaP and MiP showing normal axon paths in a STAT3 MO host. (E) Donor CaP motoneuron expressing wild-type STAT3 showing that STAT3 expression does not alter pathfinding trajectory. (G) Dominant negative STAT3 expressing CaP motoneuron showing pathfinding failure (open arrowhead) in a wild-type host. (I) Constitutively active STAT3 expressing CaP neuron shows bifurcated axon projecting both ventrally as well as along the dorsal pathway (arrow). (K) A group of donor motoneurons transplanted into a wild-type host. In no case did expression of any STAT3 variant affect MiP or RoP axon projections. The RoP and MiP pair enclosed by the dashed circle exemplifies donor neurons that were not included in any analysis since they occupy the same hemisegment and donor cell–cell interactions cannot be excluded. The lone MiP motoneuron that is labeled is the only neuron in that hemisegment originating from donor cells and was thus included in cell counts. (L) Representative donor RoP motoneuron. In condition was RoP pathfinding affected. (M–P) Expression of constitutively active STAT3 from a neurospecific promoter leads to ectopic collateral formation. (M) Normal axon projection of a CaP motoneuron expressing wild-type STAT3 from a neurospecific promoter. (O) Expression of constitutively active STAT3 produces a bifurcated axon projecting both ventrally as well as along the dorsal pathway (arrow). (N and P) ZNP1 staining of primary motoneurons present in the preceding panel. (Q–S) Schematic representation of CaP axon projections shown in the above panels. |
|
Antibody staining for phosphorylated STAT3. (A) Mosaic image of a 27 h p.f. embryo stained with an antibody to phosphorylated STAT3 (green) and with ZNP1 (red) for primary motoneurons. White arrows mark neuronal cell bodies positive for phosphorylated STAT3. Green cells out of focus are EVL cells. (B–D) Higher magnification images of neuronal soma (white arrows) possessing phosphorylated STAT3. In all images, rostral is to the left, and dorsal is up. EXPRESSION / LABELING:
|
|
Motoneuron specification is unaltered by STAT3 inactivation. Motoneuron specification was assessed by LIM homeodomain expression. In situ hybridization was performed on uninjected (A and D), STAT3 MO injected (B and E), and DN-STAT3-GFP expressing (C and F) embryos. (A–C) In situ hybridization reveals in all cases two Isl1-positive neurons per hemisegment indicating the specification of one RoP and one MiP per hemisegment. This is inconsistent with the CaP motoneuron trans-specifying into a RoP or MiP, which would result in a third Isl1-positive neuron found in a hemisegment. (D–F) In all cases, in situ hybridization for Isl2 identifies one (arrowheads) or two neurons (asterisk) per hemisegment consistent with the CaP and the variably present VaP. In all images, rostral is to the left, and dorsal is up. Embryos are at 27 h post-fertilization. EXPRESSION / LABELING:
|
|
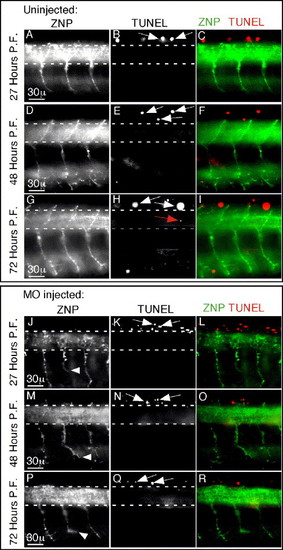
STAT3 inactivation does not induce cell death of primary motoneurons. (A–I) Uninjected embryos at indicated developmental time points were stained for apoptosis by TUNEL as well as for primary motoneurons using the ZNP1 monoclonal antibody. Up to 3 days p.f., there is little cell death in embryos. The region encompassing motoneuron soma is demarcated between dashed lines. In the spinal cord, almost all TUNEL-positive neurons are sensory Rohon-Beard neurons positioned in the dorsal spinal cord above the dashed lines (white arrows). Occasionally, an apoptotic neuron (one in eight hemisegments) was present in the region of motoneuron soma (red arrow). (J–R) STAT3 morpholino injected embryos stained as in panel A–I. There is no increased cell death associated with morpholino injection. The most ventral axon position of CaP motoneurons that failed to reach the ventral target is marked with a white arrowhead (J, M, P). Identical results were also obtained upon dominant negative DN-STAT3-GFP expression (not shown). In all images, rostral is to the left, and dorsal is up. |
Reprinted from Developmental Biology, 296(1), Conway, G., STAT3-dependent pathfinding and control of axonal branching and target selection, 119-136, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.