- Title
-
Specification of the enveloping layer and lack of autoneuralization in zebrafish embryonic explants
- Authors
- Sagerström, C.G., Gammill, L.S., Veale, R., and Sive, H.
- Source
- Full text @ Dev. Dyn.
|
The zebrafish cyt1 gene and the Xenopus XK81 gene are expressed in outer epithelial layers. A: Northern blot analysis of cyt1 expression. A cyt1 probe made from clone 1:4 (see Experimental Procedures section) was hybridized to Northern blots containing 1 μg of total RNA from various stages of development: Lane 1, 1-cell stage (3 hours postfertilization [hpf]); lane 2, sphere (4 hpf); lane 3, 50% epiboly (5 hpf); lane 4, 75% epiboly (8 hpf); lane 5, 90% epiboly (9 hpf); lane 6, tail bud (10 hpf); lane 7, 18 somites (18 hpf); lane 8, prim5 (24 hpf); lanes 9 and 10, long pec (48 hpf); lane 11, fry (1 week); lane 12, adult (3 month). Hybridization to a probe for 18S rRNA was used as a loading control. B: Whole-mount in situ hybridization analysis of cyt1 expression. A cyt1 probe (see Experimental Procedures section) was hybridized to zebrafish embryos from various stages: a, late blastula, sphere stage, 4 hpf; b, early gastrula, shield stage, 6 hpf; c, late gastrula, 85% epiboly, 9 hpf; d, mid-somitogenesis, 5-somite stage, 12 hpf; e, end of somitogenesis, prim 5, 24 hpf. Some embryos were sectioned after in situ hybridization to further define the expression of cyt1. f, section through embryo in b; g, section through embryo in c; h, section through embryo in e; i, enlargement of boxed region in h. The level of each section is indicated by a black line. Anterior is to the top in a–d and to the left in e. Dorsal is to the right in b–d and to the top in e. Dorsal cannot be assigned at the stage in a (sphere stage, 4 hpf). Dorsal is to the top in f–i. Y, yolk. C: Whole-mount in situ hybridization of XK81 expression. An XK81 probe (see Experimental Procedures section) was hybridized to Xenopus embryos from various stages; a and b, late gastrula, st. 12.5; c and d, mid-neurula, st. 15; e and f, tail bud, st. 20. Embryos were sectioned to further define XK81 expression: g, section through an early neurula stage embryo (st.14) at the level indicated in c; h, enlargement of area boxed in g; i, section through a late neurula stage embryo (st.18) at the level indicated in e; j, enlargement of boxed area in i. Anterior is to the top in a–f. a, c, and e are dorsal views. b, d, and f are lateral views. Dorsal is to the top in g–j. D, dorsal; V, ventral; A, anterior; P, posterior. |
|
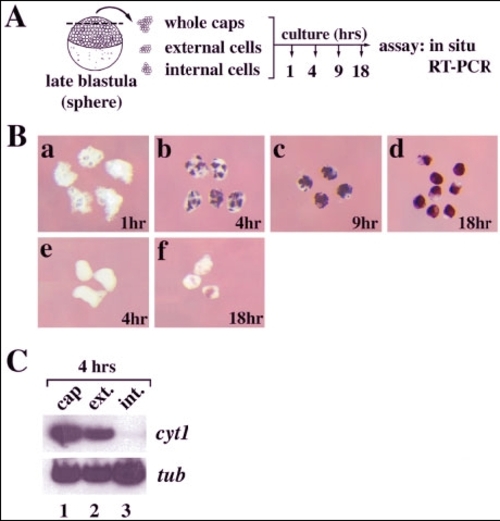
Superficially located cells are specified to express cyt1 by late blastula stages. A: Schematic outline of experiment. Animal caps were dissected from late blastula (sphere stage, 4 hours postfertilization [hpf]) stage embryos and used intact or subdivided into explants enriched for external and internal cells (see Experimental Procedures section) before culture for various periods of time. All explants were cultured in aggregates of 10. At the end of the culture period, explants were harvested and processed for in situ hybridization or reverse transcriptase-polymerase chain reaction (RT-PCR) as described in the methods section. B: Whole-mount in situ hybridization analysis of cyt1 expression. Aggregates of whole animal cap explants (a-d) or deep cell explants (e, f) were cultured for 1 hr (until 5 hpf, equivalent to early gastrula; a), 4 hr (until 8 hpf, equivalent to mid-gastrula; b, e), 9 hr (until 13 hpf, equivalent to early somitogenesis, c), or 18 hr (22 hpf, equivalent to end of somitogenesis, d, f) followed by in situ hybridization for cyt1 expression. Explants cultured for 18 hr in d showed some variability in staining and ~50% were completely covered by cyt1 staining cells. The remaining 50% showed various degrees of covering by cyt1-positive cells, but all showed some staining. None of the internal explants cultured for 4 hr in e showed staining. Although all internal explants cultured for 18 hr in f stained, the intensity of staining was highly variable, as seen in f. C: RT-PCR analysis of cyt1 expression in explants. Aggregates of whole animal cap explants (lane 1), superficial cell explants (lane 2), or deep cell explants (lane 3) were cultured for 4 hr (until 8 hpf, equivalent to mid-gastrula), harvested, and analyzed by RT-PCR for cyt1 expression. Expression of α-tubulin (tub) was used as a loading control. |
|
Dissociation of zebrafish animal caps disrupts cyt1 expression. A: Schematic overview of experimental design. Animal caps were dissected at late blastula stages (sphere stage, 4 hpf), aggregated in groups of 10 and used intact or dissociated into individual cells (see Experimental Procedures section), followed by culture for various periods of time. After the culture period, explants and cells were harvested and assayed for cyt1 expression by reverse transcriptase-polymerase chain reaction (RTPCR; see Experimental Procedures section). B: Blastula stage intact caps (lanes 1–4), dissociated caps (lanes 5–8), and whole embryos (lanes 9–12) were cultured for 2 hr (until 6 hours postfertilization [hpf], equivalent to early gastrula), 4 hr (until 8 hpf, equivalent to mid-gastrula), 6 hr (until 10 hpf, equivalent to end of gastrulation), or 8 hr (until 12 hpf, equivalent to onset of somitogenesis) before harvesting and analysis for cyt1 expression by RT-PCR. tub expression was used as a loading control. C: Blastula stage dissociated caps (lanes 1 and 2) and intact caps (lanes 3 and 4) were cultured for 4 hr (until 8 hpf, equivalent to mid-gastrula) in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 μ/ml human activin A and assayed for gsc expression by RT-PCR. tub expression was used as a loading control. |
|
Bone morphogenetic protein-4 (BMP4) does not restore cyt1 expression, and autoneuralization is not induced in dissociated zebrafish animal caps. A: Schematic representation of experiment analyzing the effect of dissociation and BMP4 treatment on gene expression in zebrafish blastula stage animal caps. Animal caps were dissected from late blastula (4 hpf, sphere stage; as shown) or early gastrula (6 hpf, shield stage; not shown), aggregated into groups of 10 and either cultured intact, or after dissociation and reassociation (see Experimental Procedures section). Explants were then cultured for 4 hr (until 8 hpf, equivalent to midgastrula)in the presence or absence of 50ng/ml BMP4 followed by analysis by reverse transcriptase-polymerase chain reaction (RTPCR)for expression ofcyt1, otx2, opl, gta3, andtubulin. B: Results of experiments on blastula stage caps. Animal caps were harvested immediately after dissection (lane 1) or after 4 hr in culture (lanes 2–4). Explants cultured for 4 hr were either dissociated into single cells and immediately reassociated (lanes 2 and 3, mins.)or left intact (lane 4). Explants were cultured in the presence (lane 3) or absence (lanes 2 and 4)of 50 ng/ml of BMP4. C: Results of experiments on gastrula stage caps. Animal caps were harvested immediately after dissection (lane 1) or after 4 hr in culture (lanes 2–5). Explants cultured for 4 hr were either dissociated into single cells and immediately reassociated (lanes 2 and3, mins.) or left intact (lanes 4 and 5). Explants were cultured in the presence (lane 3 and 5) or absence (lanes 2 and 4) of 50 ng/ml of BMP4.cyt1 is expressed in the zebrafish enveloping layer, otx2, and opl dorsally and gta3 ventrally in the zebrafish. tub was used as a loading control. EXPRESSION / LABELING:
|
|
Bone morphogenetic protein-4 (BMP4) restores XK81 expression and autoneuralization is induced in dissociated Xenopus gastrula stage animal caps. A: Schematic representation of experiment analyzing the effect of dissociation and BMP4 treatment on gene expression in Xenopus gastrula stage animal caps. Animal caps were dissected from early gastrula (st.10) and either cultured intact or after dissociation for 1 or 3 hr followed by reassociation (see Experimental Procedures section). Explants were cultured for a total of 5 hr (until st.13, equivalent to early neurula) in the presence or absence of BMP4 followed by analysis by reverse transcriptase-polymerase chain reaction (RT-PCR) for expression of XK81, otx2, opl, XVent-1, and EF1a. B: Results of experiment outlined in A. Animal caps were harvested immediately after dissection (lane 1) or after 5 hr in culture (lanes 2-6). Explants cultured for 5 hr were either dissociated into single cells and reassociated after 1 (lanes 2 and 3) or 3 (lanes 4 and 5) hr or left intact (lane 6). Explants were cultured in the presence (lane 3 and 5) or absence (lanes 2, 4, and 6) of 500 ng/ml of BMP4. XK81 is expressed in the Xenopus outer layer, otx2, and opl dorsally and Xvent-1 ventrally in Xenopus. Xenopus EF1a was used as a loading control. |

Unillustrated author statements EXPRESSION / LABELING:
|