- Title
-
Cyp27c1 Red-Shifts the Spectral Sensitivity of Photoreceptors by Converting Vitamin A1 into A2
- Authors
- Enright, J.M., Toomey, M.B., Sato, S.Y., Temple, S.E., Allen, J.R., Fujiwara, R., Kramlinger, V.M., Nagy, L.D., Johnson, K.M., Xiao, Y., How, M.J., Johnson, S.L., Roberts, N.W., Kefalov, V.J., Guengerich, F.P., Corbo, J.C.
- Source
- Full text @ Curr. Biol.
|
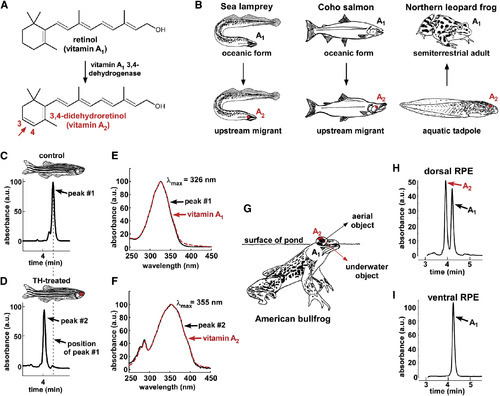
Modeling the Rhodopsin-Porphyropsin Switch
(A) The conversion of retinol (vitamin A1) to 3,4-didehydroretinol (vitamin A2) underlies the rhodopsin-to-porphyropsin switch and is driven by a previously unidentified dehydrogenase that catalyzes the addition of a double bond to the ?-ionone ring. (B) This vitamin A1 to A2 switch is widely used by a variety of vertebrates and can be deployed at key stages of the life cycle: e.g., during upstream migration in sea lamprey (Petromyzon marinus) and Coho salmon (Oncorhynchus kisutch) and upon metamorphosis in amphibians such as the northern leopard frog (Lithobates pipiens) [ 9; 10 ; 11]. (C and D) To model this switch, zebrafish were treated with thyroid hormone (300 ?g/l L-thyroxine [T4]) for 3 weeks. Retinoids were then isolated from pooled RPE and retina of three individuals, reduced using sodium borohydride, and analyzed by HPLC. Retinoids from TH-treated fish have a shorter retention time than those from vehicle-treated fish. (E) The absorbance spectrum of the predominant retinoid from control fish closely matches the absorbance spectrum of a vitamin A1 standard, with a ?max of 326 nm. (F) The predominant retinoid from TH-treated fish has an absorbance spectrum that matches that of the vitamin A2 standard, with a ?max of 355 nm. (G) The American bullfrog (Lithobates catesbeianus) often sits at the water?s surface and possesses exclusively A1-based visual pigments in the ventral retina and a mixture of A1- and A2-based visual pigments in the dorsal retina, possibly facilitating downward vision into the murky, red-shifted water [ 12 ; 13]. (H and I) The dorsal third of the RPE from two American bullfrogs was dissected, pooled, and analyzed by HPLC and found to contain a mixture of vitamin A1 and vitamin A2. Only vitamin A1 was identified in the ventral third of the RPE. All absorbance values are normalized and represented as arbitrary units (a.u.). |
|
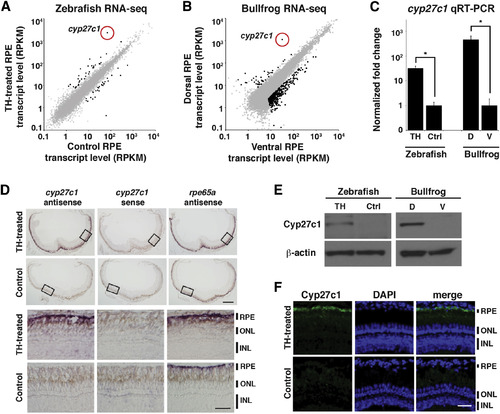
Cyp27c1 Expression Is Correlated with the Presence of Vitamin A2 in Zebrafish and Bullfrog RPE
(A) Zebrafish were treated with TH or a vehicle control for 3 weeks, after which RPE was isolated and used to construct a cDNA library for transcriptome profiling by RNA-seq. Expression levels (in RPKM [reads per kilobase of transcript per million mapped reads]) of individual transcripts from TH-treated RPE (y axis) and vehicle-treated RPE (x axis) are shown as dots, with significantly differentially expressed genes in black (quantile-adjusted conditional maximum likelihood [qCML] test; FDR < 0.05; n = 3). (B) Dorsal and ventral thirds of bullfrog RPE were isolated and used to construct a cDNA library for transcriptome profiling by RNA-seq. Expression levels of individual transcripts from dorsal RPE (y axis) and ventral RPE (x axis) are shown as dots, with significantly differentially expressed genes in black (qCML test; FDR < 0.05; n = 3). (C) Enrichment of the cyp27c1 transcript in cDNA samples used for RNA-seq was confirmed by quantitative real-time PCR (qRT-PCR). Expression was normalized to ribosomal protein rpl13a for zebrafish and rpl7a for bullfrog (two-sided Student?s t test; n = 2?3; p < 0.005; error bars = SEM). (D) In situ hybridization of albino zebrafish treated with TH or vehicle control for 3 weeks. Top panels show cross-sections of the whole eye. Bottom panels show high-magnification images of the boxed regions from the top panels. The antisense probe for cyp27c1 localized exclusively to the RPE in TH-treated fish. No signal was observed with the cyp27c1 sense probe, but strong signal was observed in the RPE of TH-treated and control fish with an antisense probe against rpe65a, a gene that is expressed at high levels in RPE. The scale bars represent 200Ám low power and 50 Ám high power. (E) Western blot with a rabbit polyclonal anti-Cyp27c1 antibody confirmed enrichment of Cyp27c1 protein in TH-treated zebrafish and dorsal bullfrog RPE. β-actin was used as a loading control. (F) Immunohistochemistry of albino TH- and vehicle-treated zebrafish indicates induction of Cyp27c1 expression in the RPE of TH-treated fish (green), with DAPI used to counter-stain nuclei (blue). The scale bar represents 50 Ám. See also Figure S1 and Tables S1 and S2. |
|
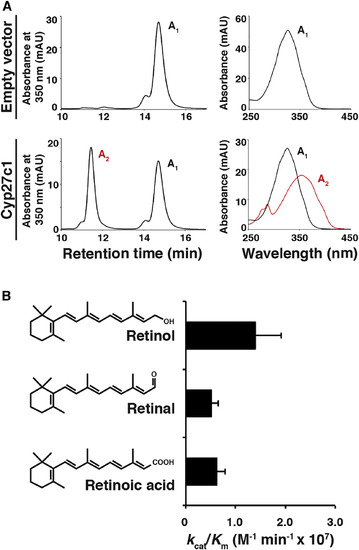
Cyp27c1 Is Sufficient to Convert Vitamin A1 into Vitamin A2
(A) HEK293 cells were transfected with a cyp27c1 expression construct or an empty vector and incubated with vitamin A1 for 24 hr, after which cells and media were harvested for HPLC. Cells transfected with empty vector did not produce vitamin A2, but cells transfected with cyp27c1 converted a substantial fraction of the vitamin A1 to vitamin A2. (B) A cell-free assay system was used to assess the catalytic efficiency of Cyp27c1 for all-trans retinol, retinal, and retinoic acid. Purified Cyp27c1 was incubated with bovine adrenodoxin (Adx), NADPH-adrenodoxin reductase (ADR), and substrate (with concentrations ranging from 0.1 to 10 ?M). The reaction was then initiated using an NADPH-generating system, run for 60 s, and quenched. HPLC was used to quantify the products in order to calculate kcat and Km. A high catalytic efficiency (kcat/Km) was observed for all three substrates. Error bars represent SEM. See also Figure S2. |
|
Cyp27c1 Is Necessary for Vitamin A2 Production and Red-Shifting of Photoreceptors In Vivo
(A) TALENs were used to generate cyp27c1 mutant zebrafish. Two independent alleles are shown, cyp27c1?1 and cyp27c1?2. Both alleles contain frame-shift mutations resulting in premature stop codons at amino acids 151 and 150, respectively. (B) Western blot with rabbit polyclonal anti-Cyp27c1 antibody shows that Cyp27c1 protein is detected in the RPE of wild-type fish, but not in the RPE of cyp27c1?1/?2 fish, after 3 weeks of TH treatment. ?-actin was used as a loading control. (C) Wild-type and cyp27c1?1/?2 siblings were treated with TH or vehicle control for 3 weeks, and retinas and RPE from three animals were pooled and analyzed for retinoid content using HPLC. A TH-driven switch to vitamin A2 was observed in wild-type fish, but not in cyp27c1?/1?2 mutants. (D) The spectral sensitivity of red cones from TH-treated cyp27c1?1?2 fish and their wild-type siblings was assessed using single-cell suction electrode recording. The photoreceptors were exposed to a series of dim flashes at 560, 600, 660, and 700 nm, and their response amplitudes were used to calculate flash sensitivity (y axis) as a function of wavelength (x axis). No significant difference was observed between vehicle-treated cyp27c1?/1?2 and wild-type red cones. However, TH-treated wild-type red cones were significantly more sensitive to 660- and 700-nm light and less sensitive to 560-nm light relative to cyp27c1?/1?2 red cones (Student?s t test; ?p < 0.0005; n = 12?21; error bars = SEM). Fitted curves were calculated using a model that incorporates both vitamin A1- and A2-based pigment. This model predicts 100% vitamin A1 in vehicle-treated wild-type and cyp27c1?/1?2 fish, 99.7% vitamin A1 in TH-treated cyp27c1?/1?2 fish, and 95.5% vitamin A2 in TH-treated wild-type siblings. (E) Physiological parameters of the red cones are indicated ▒ SEM. These include wavelength of peak sensitivity (?max), dark current (Idark), estimated half-saturation light intensity at ?max (Io), dim flash response time to peak (Tpeak), recovery time constant (?rec; estimated from a single-exponential fit to the tail of the response), and integration time (Tint; estimated as the time integral of the normalized flash response). See also Figure S3. |
|
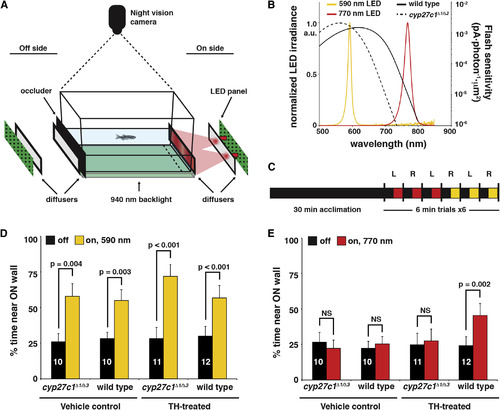
Mutation of Cyp27c1 Abolishes the Phototactic Response to Near-Infrared Light
(A) The phototaxis assay consisted of 590- or 770-nm LED light sources placed on either the right or left side of a tank (depicted on the right side in the schematic). Light from the LEDs passed through two diffusers before illuminating one end of the tank. On the opposite end of the tank, a black occluder was inserted to prevent reflection off of the ?off-side? diffuser. Fish movement was recorded using a night vision camera and a 940-nm infrared backlight located beneath the tank. The percentage of time spent within 25 mm of the lit ?on? side (dashed line) was determined. The behavioral assay and subsequent data analysis were conducted in a blinded fashion with respect to the genotype of the fish. (B) A comparison of red cone sensitivity data (reproduced from Figure 4D) and the measured LED spectra. (C) Summary of the experimental design. Each dark-adapted fish was acclimated to the testing tank for 30 min in the dark and then recorded during a set of three trials each at 770 and 590 nm. Each trial consisted of 3 min dark and 3 min lit conditions, with the order of light/dark presentation randomized. The light source was alternated between the left and right sides for each trial. (D) TH- and vehicle-treated cyp27c1?1/?3 mutants and wild-type siblings all showed a significant positive phototactic response to 590-nm light (paired Student?s t test; p < 0.005; n as indicated per group; error bars = SEM). All p values remained significant after Bonferroni correction for multiple testing. (E) Only TH-treated wild-type fish showed a significant positive phototactic response to 770-nm light. For significantly different groups, p values are as shown. For vehicle-treated cyp27c1?1/?3 mutant and wild-type fish at 770 nm, p values were 0.449 and 0.177, respectively. For TH-treated cyp27c1?1/?3 mutants at 770 nm, the p value was 0.490 (paired t test; n as indicated per group; error bars = SEM). All p values remained significant after Bonferroni correction for multiple testing. See also Figure S4 and Movies S1 and S2. |
|
TH-Treatment of Zebrafish Induces a Change in Skin Pigmentation, Related to Experimental Procedures Wild-type zebrafish are pigmented by alternating rows of melanophores and xanthophores. While this pattern is unchanged with vehicle treatment, TH-treated fish exhibit a reduction in melanophore-based pigment. This change was consistent across strains, and was used to confirm TH-treatment. |