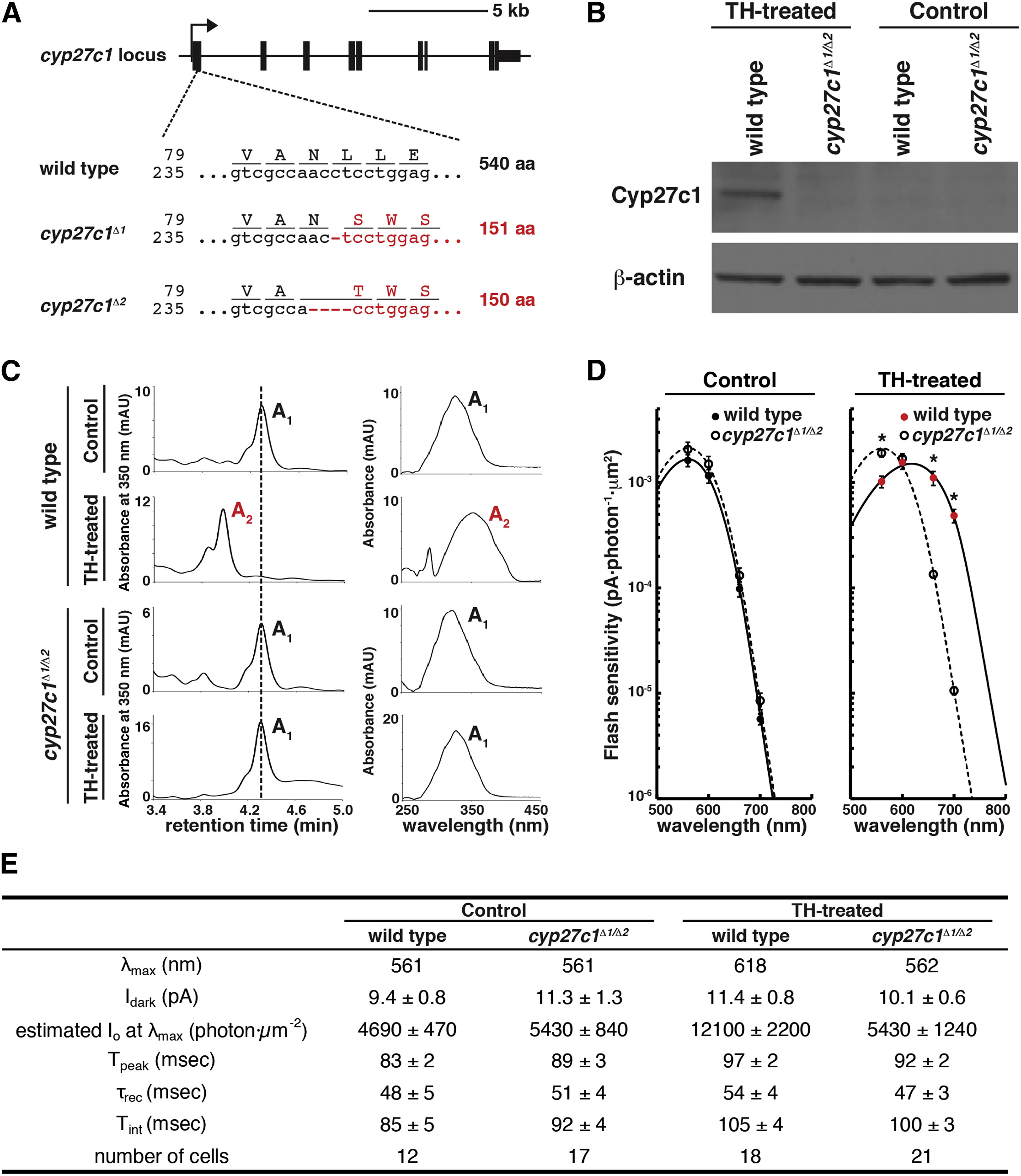
Fig. 4 Cyp27c1 Is Necessary for Vitamin A2 Production and Red-Shifting of Photoreceptors In Vivo
(A) TALENs were used to generate cyp27c1 mutant zebrafish. Two independent alleles are shown, cyp27c1Δ1 and cyp27c1Δ2. Both alleles contain frame-shift mutations resulting in premature stop codons at amino acids 151 and 150, respectively.
(B) Western blot with rabbit polyclonal anti-Cyp27c1 antibody shows that Cyp27c1 protein is detected in the RPE of wild-type fish, but not in the RPE of cyp27c1Δ1/Δ2 fish, after 3 weeks of TH treatment. β-actin was used as a loading control.
(C) Wild-type and cyp27c1Δ1/Δ2 siblings were treated with TH or vehicle control for 3 weeks, and retinas and RPE from three animals were pooled and analyzed for retinoid content using HPLC. A TH-driven switch to vitamin A2 was observed in wild-type fish, but not in cyp27c1Δ/1Δ2 mutants.
(D) The spectral sensitivity of red cones from TH-treated cyp27c1Δ1Δ2 fish and their wild-type siblings was assessed using single-cell suction electrode recording. The photoreceptors were exposed to a series of dim flashes at 560, 600, 660, and 700 nm, and their response amplitudes were used to calculate flash sensitivity (y axis) as a function of wavelength (x axis). No significant difference was observed between vehicle-treated cyp27c1Δ/1Δ2 and wild-type red cones. However, TH-treated wild-type red cones were significantly more sensitive to 660- and 700-nm light and less sensitive to 560-nm light relative to cyp27c1Δ/1Δ2 red cones (Student’s t test; ∗p < 0.0005; n = 12–21; error bars = SEM). Fitted curves were calculated using a model that incorporates both vitamin A1- and A2-based pigment. This model predicts 100% vitamin A1 in vehicle-treated wild-type and cyp27c1Δ/1Δ2 fish, 99.7% vitamin A1 in TH-treated cyp27c1Δ/1Δ2 fish, and 95.5% vitamin A2 in TH-treated wild-type siblings.
(E) Physiological parameters of the red cones are indicated ± SEM. These include wavelength of peak sensitivity (λmax), dark current (Idark), estimated half-saturation light intensity at λmax (Io), dim flash response time to peak (Tpeak), recovery time constant (τrec; estimated from a single-exponential fit to the tail of the response), and integration time (Tint; estimated as the time integral of the normalized flash response). See also Figure S3.