- Title
-
Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity
- Authors
- Sah, R., Mesirca, P., Van den Boogert, M., Rosen, J., Mably, J., Mangoni, M.E., and Clapham, D.E.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
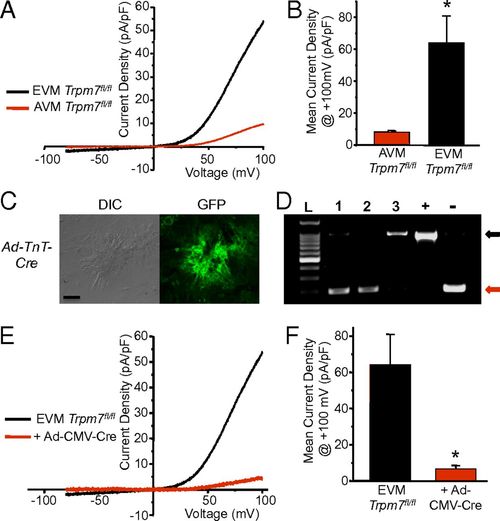
TRPM7 current in cultured murine embryonic ventricular myocytes. (A) Representative TRPM7 current measured in cultured EVM compared with AVM after full run-up. (B) Mean TRPM7 current density at +100 mV in AVM (ITrpm7, AVM = 8.3 ± 0.9 pA/pF, n = 5) compared with EVM (ITrpm7, EVM = 64.2 ± 16.7 pA/pF, n = 5). (C) Interference contrast (DIC, Left) and fluorescence GFP image (Right). (D) PCR across exon 17 from genomic DNA isolated from Trpm7fl/fl EVM transduced with Ad-TnT-Cre (1), Ad-CMV-Cre (2), and Ad-CMV-Lacz (3), Trpm7fl/fl fibroblasts (+), and Trpm7fl/- tail (-). Black arrow, full-length exon 17. Red arrow, deleted exon 17. (E) Representative TRPM7 current measured in Trpm7fl/fl EVM and Trpm7fl/fl EVM 5 d after transduction with Ad-CMV-Cre. (F) Mean TRPM7 current density in Trpm7fl/fl EVM treated with Ad-CMV-Cre (n = 3) compared with untreated Trpm7fl/fl EVM (n = 5). *P < 0.05. |
|
Trpm7 deletion in embryonic myocardium disrupts cardiac automaticity in vitro. (A) Ca2+ transients measured by high-speed line-scanning confocal microscopy in Trpm7fl/fl EVM 4–5 d after transduction with Ad-CMV-Lacz (Top), Ad-CMV-Cre (Middle), and Ad-TnT-Cre (Bottom). (B) Mean Ca2+ transient frequency, (C) cycle length, CL, (D) peak Ca2+ transients, F/Fo, and (E) Ca2+ transient length in Ad-CMV-Lacz, Ad-CMV-Cre, and Ad-TnT-Cre treated Trpm7fl/fl EVMs. *P < 0.05, **P < 0.01. |
|
Trpm7 deletion in vivo disrupts automaticity in zebrafish and induces SPs and AVB in mice. (A) (Left) Images of zebrafish embryos: water-injected (Upper) and Trpm7 MO-injected (Lower). (Right) Trpm7 MO zebrafish (n = 30) and water-injected zebrafish (n = 27) heart rates. (B) Normal sinus rhythm (p denotes P waves; atrial depolarization) with intact atrioventricular conduction in the ECG of a telemetered, conscious WT mouse. (C) Representative ECG showing an episode of SP observed in a KOαMHC-Cre mouse. Solid arrows denote location of expected p waves. (D) Representative ECGs demonstrating AVB observed in KOαMHC-Cre mice (broken black arrow, conducted QRS complexes; broken gray arrow, expected location of QRS complex). (E and F) Box plots with overlying data points showing the distribution of the frequency of (E) SPs and (F) AVB observed over 24 h of telemetric monitoring in WT (n = 7) and KOαMHC-Cre (n = 8) mice. (G) Mean heart rates of WT and KOαMHC-Cre over a 24-h period were not statistically different. In box plots, error bars represent the SD of the mean. Box height represents the SE. **P < 0.01, ***P < 0.001. PHENOTYPE:
|
|
TRPM7 is highly expressed in murine SAN. (A) Confocal images of an isolated SAN freshly dissociated from adult αMHC-Cre-ROSA26mTmG heart: differential interference contrast (Left) and fluorescence GFP image (Right). (Scale bar, 50 μm.) (B) PCR across exon 17 from genomic DNA isolated from dissected KOαMHC-Cre and WT SAN. Tail DNA from Trpm7fl/- (-) serves as a positive control for Trpm7 exon 17 deletion. Black arrow, full-length exon 17. Red arrow, deleted exon 17. (C) Representative TRPM7 current-voltage traces measured in WT SAN, WT AVM, and KOαMHC-Cre SAN. (D) Mean TRPM7 current density in WT SAN (ITrpm7, WT SAN = 32.0 ± 6.2 pA/pF, n = 6), WT AVM (ITrpm7, AVM = 8.3 ± 0.9 pA/pF, n = 5), and SAN (ITrpm7, KOαMHC-Cre SAN = 0.4 ± 0.2 pA/pF, n = 4, P < 0.01). |
|
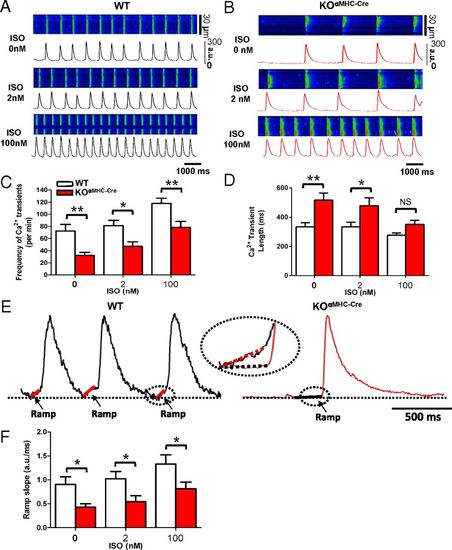
Trpm7 deleted SAN exhibit impaired automaticity with slowed diastolic Ca2+ rise. Ca2+ transients measured by high-speed line-scanning confocal microscopy in (A) WT SAN and (B) KOαMHC-Cre SAN under basal conditions (Top) and after stimulation with ISO: 2 nM (Middle) and 100 nM (Bottom). (C) Mean Ca2+ transient frequency and (D) Ca2+ transient length in WT SAN compared with KOαMHC-Cre SAN under basal conditions and after stimulation with 2 nM and 100 nM ISO. (E) Representative Ca2+ transients from WT (Left) and KOαMHC-Cre (Right) showing the slope of diastolic Ca2+ rise (Ca2+ ramp). (F) Mean slope of the diastolic Ca2+ ramp in WT and KOαMHC-Cre under increasing ISO concentrations. *P < 0.05, **P < 0.01. |
|
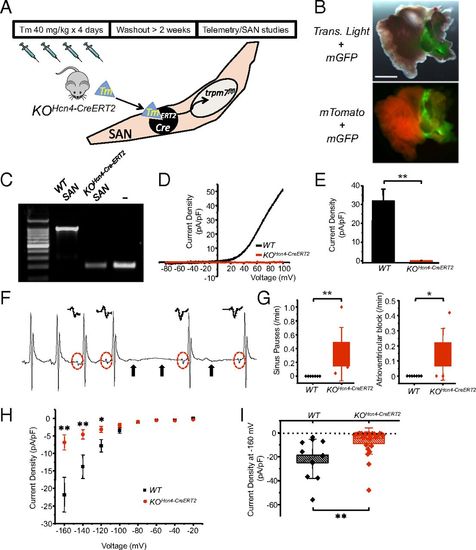
Postnatal SAN/AVN restricted Trpm7 deletion recapitulates the phenotype of global cardiac Trpm7 KO. (A) SAN-restricted Trpm7 deletion (KOHcn4-CreERT2) was achieved in Hcn4-CreERT2 × Trpm7fl/fl/Trpm7fl/- mice at 6–8 wk of age by treating with tamoxifen (40 mg/kg) by gavage for 4 d, followed by >2-wk washout period before telemetry and SAN studies (B) (Upper) Transmitted light + GFP fluorescence image of Hcn4-CreERT2-ROSA26mTmG right atrium after tamoxifen treatment. (Lower) Fluorescence mTomato/mGFP image showing recombination (mGFP) restricted to SAN region. (Scale bar, 1 mm.) (C) PCR across exon 17 from genomic DNA isolated from dissected KOHcn4-CreERT2 and WT SAN. Tail DNA from Trpm7fl/- serves as a positive control for Trpm7 exon 17 deletion (-). (D) Representative TRPM7 current–voltage traces measured in WT SAN and KOHcn4-CreERT2 SAN. (E) Mean TRPM7 current density in WT SAN and KOHcn4-CreERT2 SAN. (F) Representative ECG showing an episode of SP observed in a telemetered KOHcn4-CreERT2 mouse. Solid arrows denote location of expected p waves. Red dashed circles and Insets above show change in p-wave morphology after SP, indicative of ectopic atrial focus. (G) Box plots with overlying data points showing the distribution of the frequency SPs (Left) and AVB (Right) observed over 24 h of telemetric monitoring in WT (n = 7) and KOHcn4-CreERT2 (n = 5) mice. (H) If current–voltage relationship from WT SAN (n = 11) compared with KOHcn4-CreERT2 SAN (n = 25). (I) Box plots with overlying data points showing the mean and distribution of If current densities at 160 mV from WT SAN (n = 11) compared with KOHcn4-CreERT2 SAN (n = 25). *P < 0.05, **P < 0.01. |