- Title
-
Isolation and cytogenetic characterization of zebrafish meiotic prophase I mutants
- Authors
- Saito, K., Siegfried, K.R., Nüsslein-Volhard, C., and Sakai, N.
- Source
- Full text @ Dev. Dyn.
|
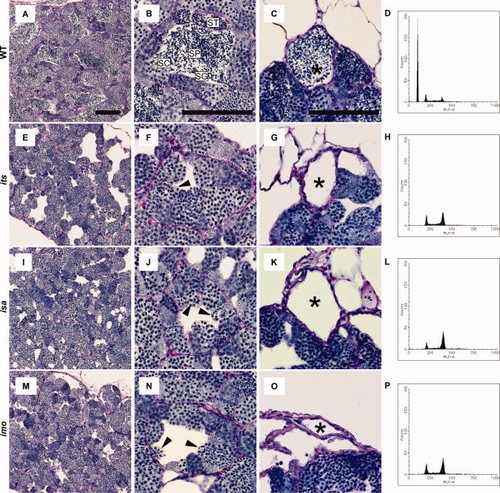
Histology and fluorescence-activated cell sorter (FACS) profiles of its, isa, and imo mutant testes. A?O: Testes sections from 3-month-old wild-type (A?C), its (E?G), isa (I?K), and imo (M?O) were stained with periodic acid Schiff and hematoxylin. A,E,I,M: Gross appearance of wild-type and mutant testes. Mutant tubules show gaps in the intertubular space. B,F,J,N: Seminiferous tubules in wild-type and mutant showing spermatogenic cysts containing different types of germ cells; spermatogonia (SG), spermatocytes (SC), spermatids (ST), and sperm (SP). Note the absence of spermatids and sperm and the presence of germ cells with condensed nuclei in the mutant testes (arrowheads). C,G,K,O: The efferent ducts of each mutant contain no sperm. Scale bars = 100 μm. D,H,L,P: Representative FACS profiles in the wild-type (D), its (H), isa (L), and imo (P) testis. Cell types in the various peaks are (1C) sperm and spermatids; (2C) spermatogonia, secondary spermatocytes, and somatic cells; and (4C) primary spermatocytes and G2/M spermatogonia and somatic cells. Each panel is representative of three zebrafish with data. Note the absence of 1C and the increase in 2C and 4C cells in each mutant testis, compared with the wild-type testis (D). PHENOTYPE:
|
|
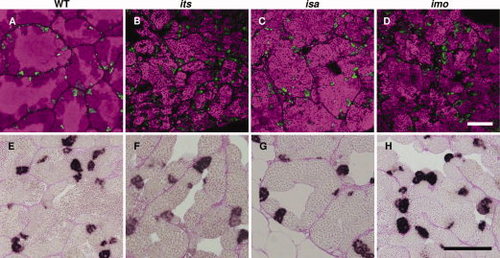
Characterization of premeiotic germ cells in the its, isa, and imo zebrafish mutants. A?D: Immunohistochemical analysis of Plzf. Fresh-frozen sections of wild-type (A), its (B), isa (C), and imo (D) testes were stained with anti-Plzf antibodies (green). The nuclei stained with TOPRO-3 are visible in a magenta pseudo-color. Plzf-expressing male zebrafish germ cell populations, which contain type A spermatogonia and early type B spermatogonia, were normal in terms of cell number and distribution patterns in each mutant. E?H: Analysis of cell proliferation by BrdU (5-bromo-22-deoxy-uridine) incorporation. Zebrafish were labeled with BrdU for 2 hr and testicular paraffin sections were subsequently stained with anti-BrdU antibodies and counterstained with periodic acid?Schiff. In the wild-type zebrafish testis, cysts of BrdU-positive type B spermatogonia, which are premeiotic spermatogenic cells, were observed. In the testis of the its (F), isa (G), and imo (H) mutants, the incorporation of BrdU in type B spermatogonia were also observed. Scale bars = 100 μm. |
|
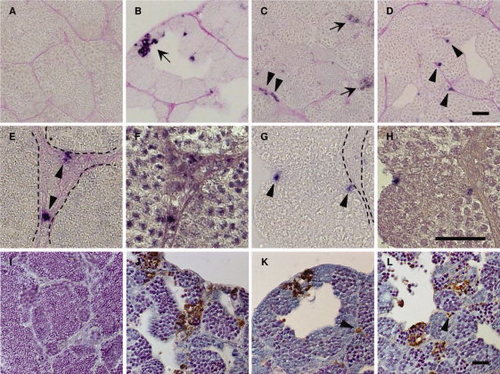
Apoptotic cells in the its, isa, and imo mutant testes. A?H: Immunohistochemical staining of cleaved Caspase-3 in wild-type (A), its (B), isa (C, E, and F), and imo (D,G,H) testes. The testes sections were stained with anti-cleaved Caspase-3 antibodies, and counterstained with periodic acid Schiff. Immunological reactivity was observed in germ cells in its (B), both germ cells and Leydig cells in isa (C), and Sertoli cells in imo (D). High magnification view of hematoxylin counterstaining images (F,H) are same position as seen in cleaved Caspase-3 positive cells in isa (E) and in imo (G). Dotted line indicates the position of the basement membrane. Arrows indicate apoptotic germ cells (B and C). Arrowheads indicate apoptotic Leydig cells (C,E) and Sertoli cells (D,G), respectively. I?L: TUNEL (terminal deoxynucleotidyl transferase?mediated deoxyuridinetriphosphate nick end-labeling) staining of wild-type (I), its (J), isa (K), and imo (L) testes. Wild-type testis shows a very low incidence of apoptosis. In contrast, the high incidence of apoptotic TUNEL staining (brown) was observed in germ cells of all three mutant testes. In addition, apoptotic TUNEL staining was also observed in Leydig cells (K) and Sertoli cells (L). Arrowheads indicate apoptotic Leydig cells (K) and Sertoli cells (L), respectively. Scale bars = 25 μm. PHENOTYPE:
|
|
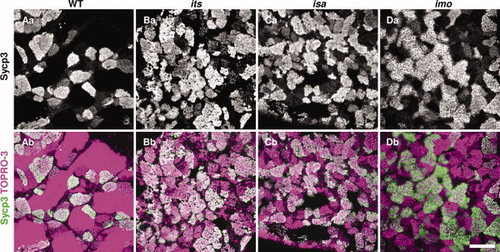
Immunohistochemical analyses of Sycp3 in the its, isa, and imo mutant testes. Fresh-frozen testes sections from wild-type (A), its (B), isa (C), and imo (D) zebrafish were stained with anti-Sycp3 antibodies (white in upper panels, green in lower panels), and counterstained with TOPRO-3 (magenta). Merged images with counterstaining are shown in the bottom panels. Sycp3 is a structural component of the lateral element of the synaptonemal complex, and is an established meiotic prophase I marker. Note the higher incidence of stained cells in the mutant testis compared with wild-type, indicating that a higher number of spermatocytes has accumulated during meiotic prophase I. Scale bar = 100 μm. |
|
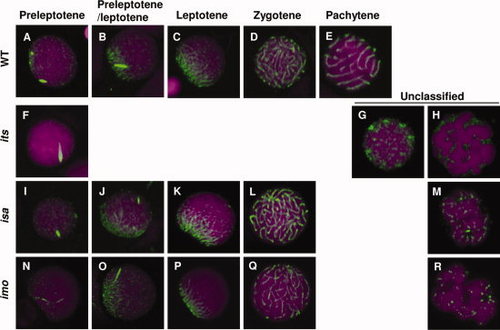
Immunocytochemical analyses of Sycp3 in spermatocytes from the its, isa, and imo mutants. Spermatocytes from wild-type (A?E), its (F?H), isa (I?M), and imo (N?R) were stained with anti-Sycp3 antibodies (green) and counterstained with TOPRO-3 (magenta). Each developmental stage of the spermatocytes was identified according to their synaptonemal complex (SC) structures. A?E: Localization of Sycp3 in wild-type spermatocytes at the preleptotene (A), leptotene?zygotene transition (B), leptotene (C), zygotene (D), and pachytene (E) stages. F?H: Localization of Sycp3 in its spermatocytes. The most progressed its spermatocytes were similar to wild-type spermatocytes at the preleptotene stage (F). Two types of abnormality were encountered; an accumulation of dotted SCs throughout the nucleus (G) and highly condensed chromosomes (H). I?M: Localization of Sycp3 in isa spermatocytes. L: The most progressed isa spermatocytes were similar to those of the wild-type zygotene stage. M: The most frequently encountered abnormal cells were associated with short fragments of the SC throughout the nucleus. N?R: Localization of Sycp3 in imo spermatocytes. Q: The most progressed imo spermatocytes were similar to wild-type zygotene stage spermatocytes. R: The most frequently encountered abnormal cells were associated with short fragments of the SC and slightly condensed chromosomes throughout the nucleus. |
|
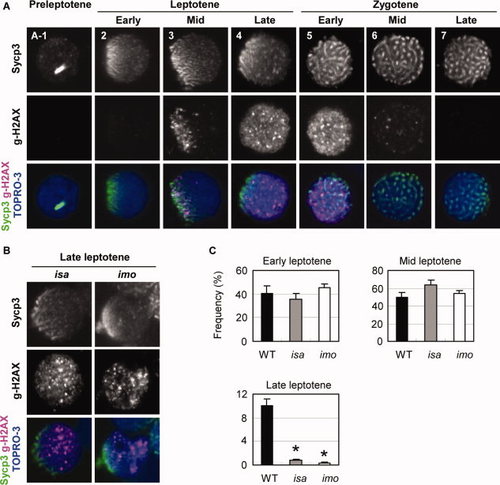
Expression and localization of γ-H2AX in isa and imo mutant spermatocytes. A: Double immunocytochemical analysis of Sycp3 (upper) and γ-H2AX (middle), and counterstaining with TOPRO-3 (blue) in wild-type spermatocytes. No γ-H2AX signals were detected in preleptotene (A-1) and early leptotene (2) cells. (3) At the mid-leptotene stage, γ-H2AX first appeared to be restricted and polarized at the nuclear region in which Sycp3 was clustered. (4) In the late leptotene stage, the γ-H2AX domains are scattered throughout the nucleus as massive accumulations. (5) Early zygotene stage. (6) Mid zygotene stage. (7) Late zygotene stage. γ-H2AX labeling has disappeared. B: Substantial reduction of normal late leptotene spermatocytes in the isa and imo mutants. Mutant leptotene spermatocytes exhibit a severe impairment of γ-H2AX expansion throughout the nucleus. Only a few late leptotene spermatocytes with a scattered expression of γ-H2AX were detectable in isa and imo. C: Quantification of spermatocytes in early, mid, and late leptotene stage spermatocytes. Late leptotene spermatocytes are significantly decreased in mutants. The values shown are the mean ± SEM of 200 leptotene spermatocytes from three zebrafish in each case. Statistical comparisons with wild-type were performed at a given leptotene stage using the Student′s t-test; *P < 0.05. |

Unillustrated author statements PHENOTYPE:
|