- Title
-
Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements
- Authors
- Lai, S.L., Chan, T.H., Lin, M.J., Huang, W.P., Lou, S.W., and Lee, S.J.
- Source
- Full text @ PLoS One
|
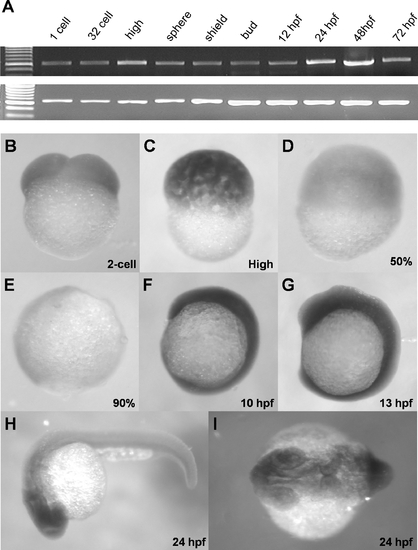
Ubiquitous expression of zdia2 during early embryonic development. (A) Expression of a 578-bp fragment of zdia2 at different embryonic stages was examined by RT-PCR, and the transcription of a 717-bp β-actin fragment was used as an internal control. (B-I) WISH of zdia2 showed ubiquitous expression in the entire embryo during early stages, but the transcripts had enriched in the head region at 24 hpf. The riboprobe was synthesized from a 1487-bp fragment beginning at the start codon of zdia2. EXPRESSION / LABELING:
|
|
Knockdown of zdia2 by MO interferes with gastrulation cell movements in a dose-dependent manner. Embryos injected with 8 ng of standard control (std) MO (the upper row) and zdia2 sMO (the bottom row) were examined and photographed under a stereomicroscope at 10 hpf. Embryos were then fixed, stained by a no tail riboprobe (C, D, K and L, side view; E and F, vegetal view; I and J, dorsal view) or in combination with a goosecoid riboprobe and photographed (K and L, side view). Tail buds and notochords are respectively indicated by black and white arrowheads (C–F). The zdia2 sMO caused incomplete epiboly formation with a ring structure near the vegetal pole as indicated by the ntl staining (D and F, open arrowheads). Widths of the notochords were measured and labeled in I and J. Lengths of the body axis were also estimated by the angle (labeled) between the prechordal plate and tail bud as described in the text (K and L). (M) Different MOs at 4 or 8 ng as designated were injected, and the embryos were examined at 10 hpf. Each treatment was repeated at least three times and analyzed as described, and only upper error bars of standard deviations are shown. (N) Embryos were injected with stdMO, zdia2 sMO or zdia2 sMO with 50 pg mdia mRNA as indicated. Epiboly and convergent extension defects were determined, analyzed, and presented as described in (M). Different letters on top of each column indicate a significant difference between treatments (P<0.05). |
|
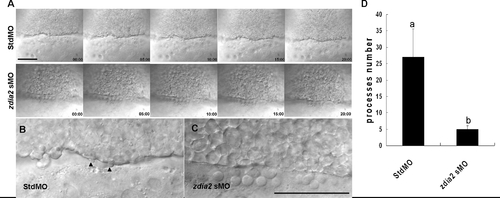
Knockdown of zdia2 inhibits epiboly cell migration by inhibiting cell protrusion formation. Embryos injected with the standard control MO (stdMO) or the zdia2 splice-blocking MO (sMO) were dechorionated, immobilized, monitored, and recorded on 20-minutes continuous time-lapse movies (See supplement movie S1 and S2 for the stdMO and sMO-injected embryo, respectively). Photographs from each recording at 5-minutes intervals are shown in (A) with the recording time given in the lower right corner. All photographs are positioned with animal pole up. In the magnified pictures, marginal deep cells of the stdMO-injected embryo (B) exhibited clear protrusive activity with two protruded cell processes indicated by arrowheads, but no such protrusions were evident in the zdia2 sMO-treated embryo (C). Bars indicate 100 μm. (D) Cell protrusions formation at the leading edge of marginal deep cells in both stdMO- and zdia2 sMO-injected embryos were quantified separately by counting protrusions formed within the first 50 frames form 3 different movies, respectively. Results were then analyzed and only upper error bars of standard deviations are shown. Different letters on top of each column indicate a significant difference between treatments (P<0.05). PHENOTYPE:
|
|
Knockdown of zdia2 inhibits cell protrusion formation of prechordal plate (primordial) cells and lateral (mesendodermal) cells. (A) Embryos injected with the standard control MO (stdMO) or the zdia2 splice-blocking MO (sMO) were dechorionated, immobilized, monitored, and recorded on 10-minutes continuous time-lapse movies. (B) Protrusions formation of cells on focus in both stdMO- and zdia2 sMO-injected embryos were quantified separately by counting protrusions formed within the first 10 minutes form at least 3 different movies, respectively. Results were then analyzed and only upper error bars of standard deviations are shown. Different letters on top of each column indicate a significant difference between treatments (P<0.05). PHENOTYPE:
|
|
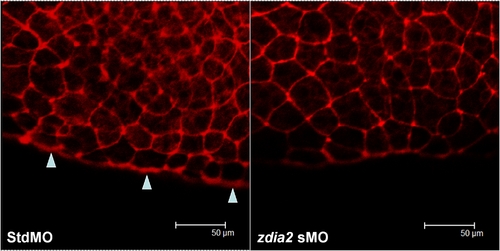
Knockdown of zdia2 inhibit actin condensation at the front edge of blastoderm. Embryos injected with 8 ng stdMO and zdia2 sMO were fixed at the germ-ring stage and photographed under confocal microscope after rhodamine phalloidin staining. Filamentous actins (arrowheads) were enriched at the front edge of the epiboly marginal deep cells of the stdMO-injected embryo (A), but not in the zdia2 sMO treated one (B). PHENOTYPE:
|
|
zdia2 function is required cell-autonomously for the cell protrusions and migration. Rhodamine-labeled cells were transplanted from embryos injected with 8 ng StdMO (A) and 8 ng zdia2 sMO (B) with Q-rhodamine, respectively, to wild type embryos. (C) Curvillinear velocity (Vcl) and strait line velocity (Vsl) were calculated and quantified (p<0.05). |
|
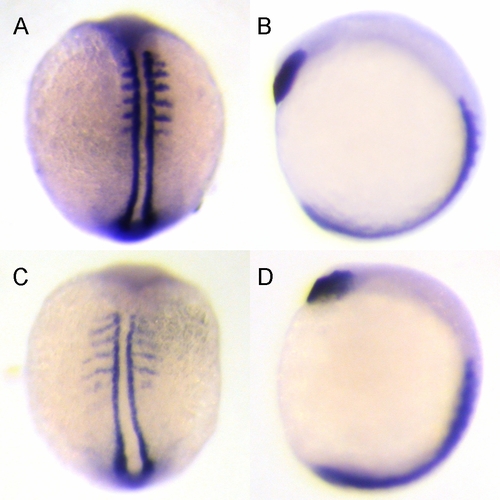
Knockdown of zdia2 by MO interferes with convergent extension cell movements. Embryos injected with 10 ng zdia2 sMO (A and B) or 10 ng stdMO (C and D) were fixed at 6–8 somite stage and stained with myoD and hatching gland riboprobes by WISH. Photographs were taken from dorsal view (A and C) and side view (B and D). EXPRESSION / LABELING:
PHENOTYPE:
|
|
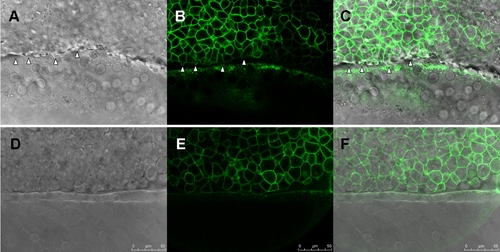
Knockdown of zdia2 interferes with protrusion formation at marginal deep cells during epiboly cell movement. Embryos injected with stdMO (A–C) or zdia2 sMO (D–F) and GFP-GAP43 mRNA were observed under confocal microscope at the 50% epiboly to shield stage. Movies with 15 frame per sec were recorded and selected snapshots from one of the stdMO-injected and zdia2 sMO-injected embryos movies with DIC channel (A and D), GFP channel (B and E) and overlap of two channels (C and F) are showed here. Blebbing cell processes are indicated by arrowheads. |
|
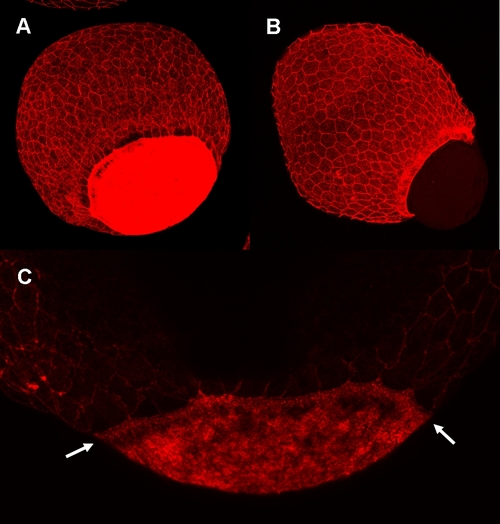
Knockdown of zdia2 inhibit actin condensation at the YSL. Embryos injected with 8 ng stdMO (A and C) and zdia2 sMO (B) were fixed at the germ-ring stage and photographed under confocal microscope after rhodamine phalloidin staining. Ring-like actin condensation (arrows in C) were at the YSL of the stdMO-injected embryo (A and C), but not in the epiboly-defected zdia2 morphant (B). PHENOTYPE:
|
|
Synergistic effect of zdia2 sMO and profilin I tMO. Embryos were injected with 8 ng zdia2 sMO (A and B) or co-injected with 4 ng zdia2 sMO and 4 ng profilin I tMO (C and D). Embryos were incubated until tail bud stage (10 hpf), fixed and stained with ntl and gsc riboprobe. Photographs were taken for side view (A and C) and dorsal view (B and D) after WISH. |

Unillustrated author statements PHENOTYPE:
|