Fig. 1
- ID
- ZDB-FIG-250721-20
- Publication
- Müller et al., 2025 - Ena/VASP-EVH1 inhibition prevents chemotaxis and metastasis by blocking the EVH1-WAVE2 interaction
- Other Figures
- All Figure Page
- Back to All Figure Page
|
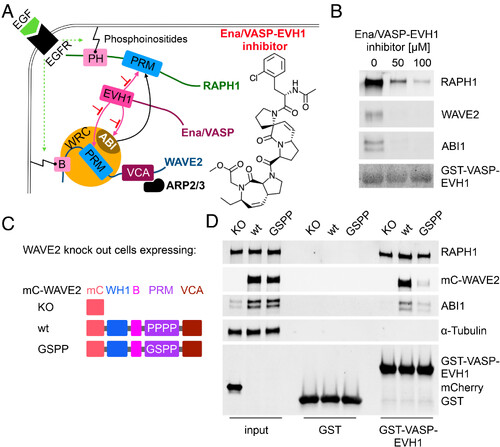
WAVE2 binds with LPPPP motif to EVH1. (A) Schematic illustration of the Ena/VASP-EVH1 interactome at the leading edge. Epidermal growth factor receptor (EGFR) signaling leads to phosphoinositides-mediated membrane association of RAPH1 via the pleckstrin-homology (PH) domain and WAVE2 via the basic (B) region. The proline-rich motif (PRM) of RAPH1, and the WAVE regulatory complex (WRC) members WAVE2, and ABI interact with the Ena/VASP-EVH1 domain and interfere with the EVH1 inhibitor. WAVE2- verprolin homology/central/acidic (VCA) domain activates the ARP2/3 complex, enabling actin polymerization and leading-edge protrusion. (B) Pull-down assays with bead-immobilized GST-VASP-EVH1 using MDA-MB-231 cell lysate and Ena/VASP-EVH1 inhibitor. The Experiment was performed in two independent replicates. (C) WAVE2 constructs: WASP homology 1 domain (WH1), basic region (B), PRM, VCA, mCherry (mC), knockout (KO). (D) Pull-down assays with bead-immobilized GST-VASP-EVH1 or GST alone as a control using cell lysates from WAVE2KO, mC-WAVE2wt, or mC-WAVE2GSPP cells. Input indicates equal expression rates for both WAVE2 constructs (panel mC-WAVE2, lanes 2 and 3) and mCherry (Lower panel, lane 1). Results were verified in two independent assays and representative results are shown. |