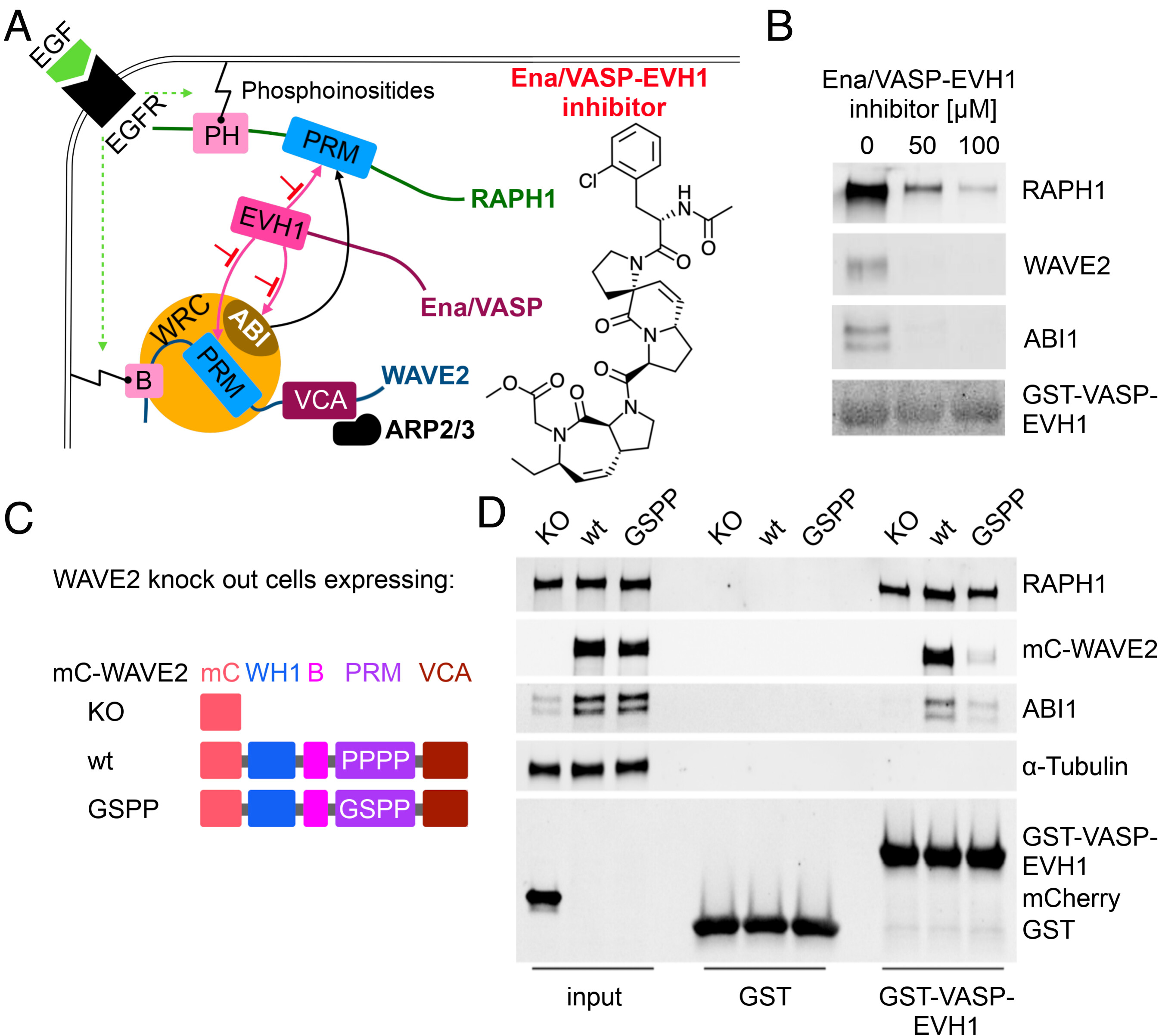
Fig. 1 WAVE2 binds with LPPPP motif to EVH1. (A) Schematic illustration of the Ena/VASP-EVH1 interactome at the leading edge. Epidermal growth factor receptor (EGFR) signaling leads to phosphoinositides-mediated membrane association of RAPH1 via the pleckstrin-homology (PH) domain and WAVE2 via the basic (B) region. The proline-rich motif (PRM) of RAPH1, and the WAVE regulatory complex (WRC) members WAVE2, and ABI interact with the Ena/VASP-EVH1 domain and interfere with the EVH1 inhibitor. WAVE2- verprolin homology/central/acidic (VCA) domain activates the ARP2/3 complex, enabling actin polymerization and leading-edge protrusion. (B) Pull-down assays with bead-immobilized GST-VASP-EVH1 using MDA-MB-231 cell lysate and Ena/VASP-EVH1 inhibitor. The Experiment was performed in two independent replicates. (C) WAVE2 constructs: WASP homology 1 domain (WH1), basic region (B), PRM, VCA, mCherry (mC), knockout (KO). (D) Pull-down assays with bead-immobilized GST-VASP-EVH1 or GST alone as a control using cell lysates from WAVE2KO, mC-WAVE2wt, or mC-WAVE2GSPP cells. Input indicates equal expression rates for both WAVE2 constructs (panel mC-WAVE2, lanes 2 and 3) and mCherry (Lower panel, lane 1). Results were verified in two independent assays and representative results are shown.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Proc. Natl. Acad. Sci. USA