Fig. 4
- ID
- ZDB-FIG-241104-33
- Publication
- Song et al., 2024 - Thymosin β4 promotes zebrafish Mauthner axon regeneration by facilitating actin polymerization through binding to G-actin
- Other Figures
- All Figure Page
- Back to All Figure Page
|
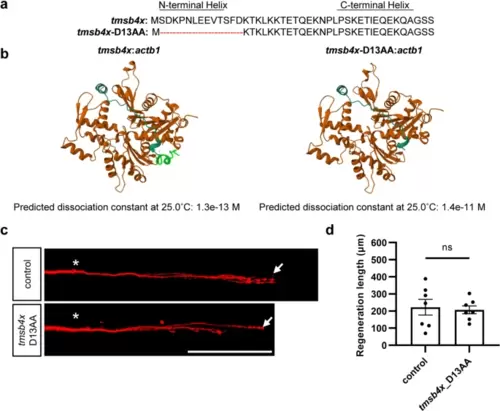
The 13 N-terminal amino acids of Tβ4 are essential for its axon regeneration-promoting activity. a Schematic representation of the protein sequence of tmsb4x and the mutant protein sequence with a deletion of the 13 N-terminal amino acids. b 3D models of wild-type (left) and N-terminal 13 amino acids deleted (right) Tβ4 in complex with β-actin, predicted by AlphaFold2. In the left image, the N-terminal 13 amino acids forming the α-helix are highlighted in green. Below the images are the dissociation constants predicted by PRODIGY based on the 3D models. c and d Representative images (c) and statistical analysis (d) of axon regeneration in zebrafish overexpressing mutant tmsb4x with deletion of the 13 N-terminal amino acids (control, 222.5 ± 45.5 μm, n = 7; tmsb4x_D13AA, 207.1 ± 23.1 μm, n = 7). The results were assessed by an unpaired, two-tailed t test, p = 0.77. The asterisks indicate the sites of injury, and the arrows indicate the terminals of the regenerated axon. Scale bar, 100 μm |