Fig. 4
- ID
- ZDB-FIG-240910-37
- Publication
- García-Cuesta et al., 2024 - Allosteric modulation of the CXCR4:CXCL12 axis by targeting receptor nanoclustering via the TMV-TMVI domain
- Other Figures
- All Figure Page
- Back to All Figure Page
|
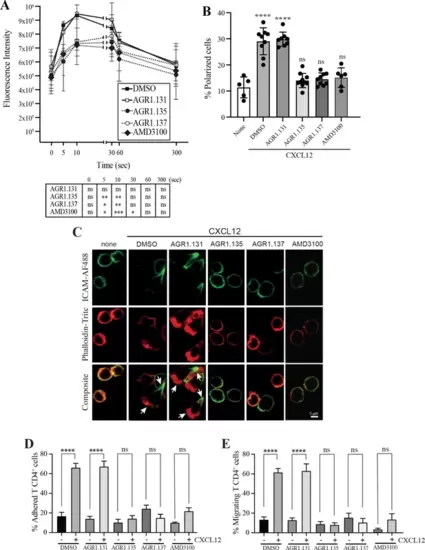
AGR1.135 and AGR1.137 treatment alter CXCL12-mediated actin polymerization. (A) Actin polymerization in response to CXCL12 as determined by F-actin (phalloidin-TRITC) staining in Jurkat cells treated with DMSO (vehicle) or the indicated modulators. Statistical significance of the different time points in comparison with the control (DMSO) is shown in the table (mean ± SD; n=3; n.s. not significant; *p≤0.05, **p≤0.01, ***p≤0.001). (B) Percentage of polarized T cell blasts adhered to fibronectin and treated or not with CXCL12 in the presence of the indicated antagonists, as analyzed by immunostaining with anti-ICAM3-Alexa fluor 488 and phalloidin-TRITC. More than 500 cells were analyzed in each condition. Data are presented as percentage of polarized cells (mean ± SD; n=3; n.s. not significant; ****p<0.0001). (C) Representative images of T cell blasts adhered to fibronectin and treated or not with CXCL12 in the presence of the indicated antagonists, as analyzed by immunostaining with anti-ICAM3-Alexa fluor 488 and phalloidin-TRITC as in B. (D) CD4+ T cells pretreated with AGR1.131, AGR1.135 or AGR1.137 were perfused in flow chambers coated with ICAM-1-containing lipid bilayers, alone or CXCL12-coated, and analyzed for cell contacts with the substrate. Data are presented as percentage of adhered cells (mean ± SD; n=3; n.s. not significant; ****p≤0.0001). (E) Cells in D were analyzed for cell migration. Data are presented as percentage of migrating cells (mean ± SD; n=3; n.s. not significant; ****p≤0.0001). |