|
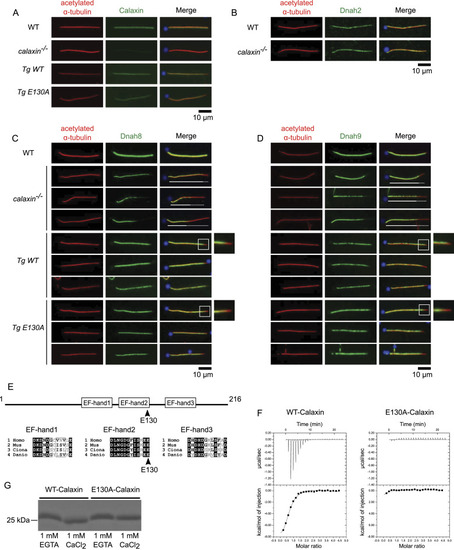
Calaxin calcium-binding activity is dispensable to stabilize outer arm dynein (OAD). (A, B, C, D) Immunofluorescence of WT, calaxin−/−, Tg(actb2:calaxin);calaxin−/−(Tg WT), and Tg(actb2:calaxin_E130A);calaxin−/−(Tg E130A) sperm. Sperm were stained with anti-Calaxin (A), Dnah2 (B), Dnah8 (C), or Dnah9 (D) antibodies (green) and costained with DAPI (blue) and acetylated α-tubulin (red). Scale bars, 10 μm. (A)calaxin−/− sperm flagella did not contain Calaxin, whereas Tg WT and Tg E130A restored this phenotype. (B)calaxin−/− sperm flagella contained Dnah2 as in WT. (C, D)calaxin−/− sperm flagella lacked Dnah8 and Dnah9 on their distal half, whereas WT sperm contained them along the whole length of the axoneme. The amount of Dnah8 and Dnah9 was variable between calaxin−/− flagella. White lines and dotted lines show OAD(+) and OAD(−) regions, respectively. Tg WT and Tg E130A contained Dnah8 and Dnah9 along the length of the axoneme except for distal tips (white boxes), almost restoring the phenotype of the calaxin−/− mutant. The magnified images of distal tips (white boxes) are also shown on the right. (E) Domain structure of Calaxin (top) and multiple alignments of three EF-hand calcium-binding regions (bottom). E130 (arrowheads) is the last residue of the EF-hand2, which is the most highly conserved between Homo sapiens, Mus musculus, Ciona intestinalis, and Danio rerio. (F) Isothermal titration calorimetry of recombinant WT-Calaxin (left) and E130A-Calaxin (right). Three sequential binding site models were used for fitting. WT bound to three calcium ions per molecule, whereas E130A showed no binding. (G) SDS–PAGE of recombinant WT-Calaxin and E130A-Calaxin with 1 mM EGTA or CaCl2. WT-Calaxin showed higher mobility in 1 mM CaCl2 compared with 1 mM EGTA, whereas E130A-Calaxin showed a slight difference.
|