- Title
-
Calaxin is a key factor for calcium-dependent waveform control in zebrafish sperm
- Authors
- Morikawa, M., Yamaguchi, H., Kikkawa, M.
- Source
- Full text @ Life Sci Alliance
|
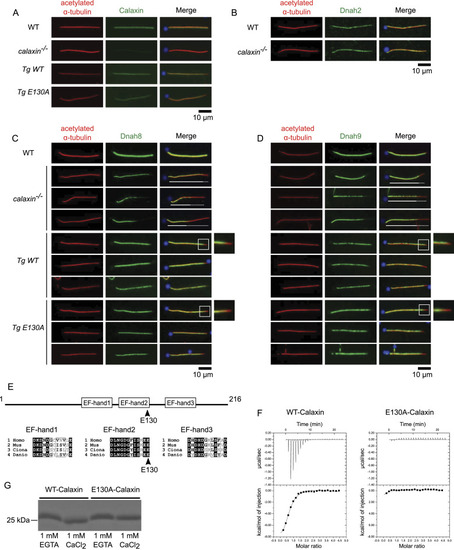
Calaxin calcium-binding activity is dispensable to stabilize outer arm dynein (OAD). |
|
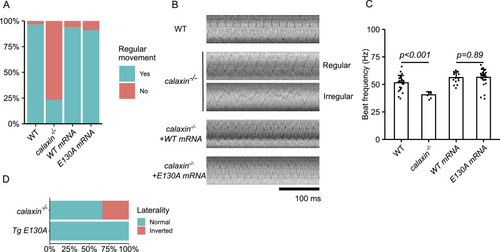
Calaxin calcium-binding activity is dispensable for KV ciliary movement. |
|
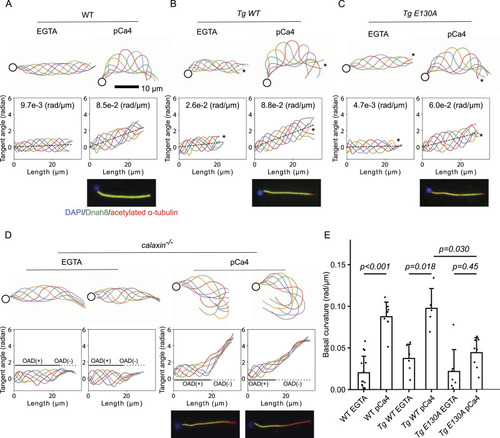
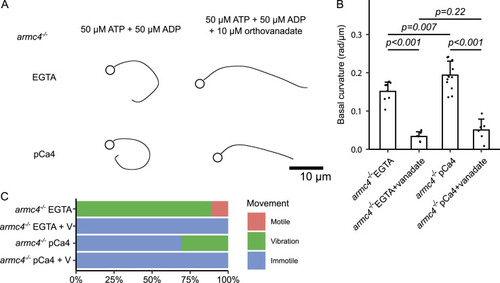
Calaxin calcium-binding activity is necessary for the calcium-induced asymmetric beating of sperm. |
|
Recombinant WT- and E130A-Calaxin can bind to a Calaxin-deficient outer arm dynein (OAD). Immunofluorescence of sperm flagella from WT, |
|
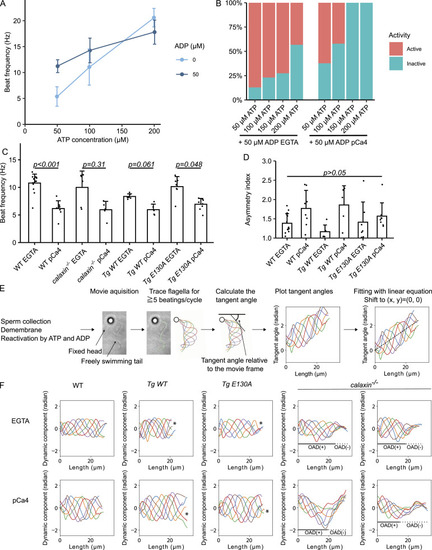
Sperm model reconstituted flagellar beating. |
|
Calcium increased the asymmetry of |