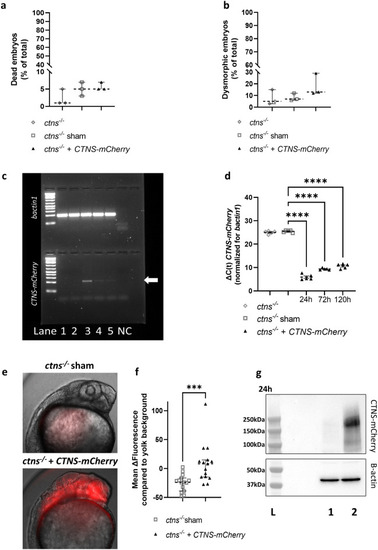
Injection of human CTNS-mCherry mRNA in fertilized eggs of ctns−/− zebrafish results in embryonic protein expression and does not cause toxicity. (a,b) The percentage (%) of dead (a) and dysmorphic embryos (b) was quantified at 5 days post-injection of human CTNS-mCherry (30–150 embryos per experiment, n = 3). Statistical analysis was done by means of a Kruskal Wallis test. Median and 95% CI are indicated. (c,d) CTNS-mCherry mRNA levels were quantified in the untreated (lane 1) and sham injected (lane 2) fish at 24 h (lane 3), 72 h (lane 4) and 120 h (lane 5) post-injection by quantitative RT-PCR (d) and agarose gel electrophoresis (arrow—c). Expression of bactin1 mRNA was used for normalization. Per condition, ΔC(t) is shown for each biological replicate (n = 5) and analysis was done with an one way ANOVA. Untreated and sham group C(t)s were artificially put at 40 cycles for quantification and mean and SEM are shown. ****p < 0.0001. (e,f) Protein expression was quantified at 24 h post-injection by live in vivo microscopy. Mean mCherry-fluorescence per embryo was quantified in a total of 15 embryos and the yolk background subtracted. Images were obtained from the larvae head region using the Olympus IX71 widefield fluorescence microscope. Statistical analysis was performed with a Mann–Whitney test and median and 95% CI are indicated. ***p < 0.001. (g) Expression of cystinosin-mCherry protein at 24 h was confirmed in CTNS-mCherry injected fish (lane 2) in comparison with the sham injected control (lane 1) by a western blot (top) with beta-actin as the loading control (bottom). Original blots and gel are presented in Supplementary Figs. S4 and S5. L=ladder.
|

