Figure 4—figure supplement 1.
- ID
- ZDB-FIG-231013-15
- Publication
- Simon et al., 2023 - Estimating the true stability of the prehydrolytic outward-facing state in an ABC protein
- Other Figures
- All Figure Page
- Back to All Figure Page
|
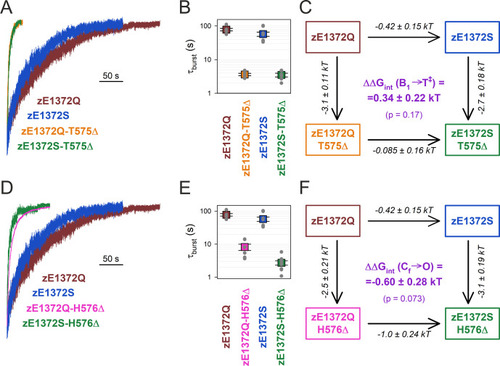
No coupling between positions 575/576 and 1372 of zCFTR E1372Q in the bursting state. (A, D) Macroscopic current relaxations following ATP removal for indicated zCFTR channel mutants (color coded). Experiments were performed as in Figure 3A, and current amplitudes are shown normalized by their steady-state values in ATP (i.e., just before ATP removal). (B, E) Relaxation time constants of the currents in (A, D), obtained by fits to single exponentials. Data are shown as mean ± standard error of the mean (SEM) from 6 to 10 experiments using a logarithmic ordinate. (C, F) Thermodynamic mutant cycles showing mutation-induced changes in the height of the free enthalpy barrier for the B1 → IB1 transition (ΔΔG0T‡−B1, numbers on arrows; k, Boltzmann’s constant; T, absolute temperature). Each corner is represented by the mutations introduced into positions Q1372 and either T575 (C) or H576 (F) of E1372Q-zCFTR. ΔΔGint(B1 → T‡) (purple number) is obtained as the difference between ΔΔG0T‡−B1 values along two parallel sides of the cycle. |