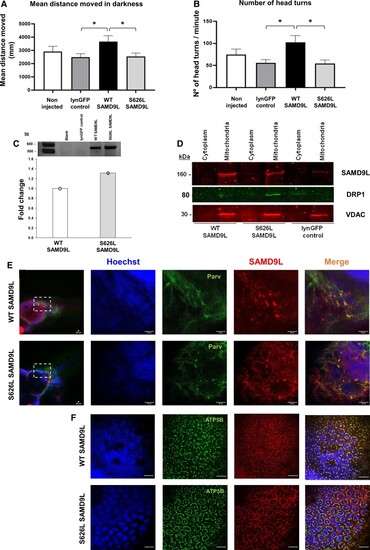
Mutant Ser626Leu SAMD9L triggers locomotive and neurosensory impairment and co-localizes with ATP5B or parvalbumin in zebrafish neurons. (A) One-way ANOVA with the mean distance travelled (mm) during five dark periods of high activity revealed significantly increase in the WT-SAMD9L zebrafish (N = 26) compared with the lynGFP control group (N = 28) [F(1,52) = 6.583, P = 0.013], and significantly decreased in S626L-SAMD9L zebrafish (N = 24) compared with the WT-SAMD9L group [F(1,48) = 4.745, P = 0.034]. (B) The number of head turns during five light–dark cycles of high activity was significantly increased in WT-SAMD9L compared with the control group (Mann–Whitney U = 216, P = 0.010), and significantly decreased in S626L–SAMD9L zebrafish larvae compared with the WT-SAMD9L (Mann–Whitney U = 192.5, P = 0.022), indicative of vestibular and sensory impairment in mutant animals. (C) SAMD9L cDNA expression did not show differences between SAMD9L-WT and SAMD9L-S626L groups compared by qRT-PCR, meaning a pool of five embryos for each group; three technical replicates (three values/group) each for SAMD9L normalized to zebrafish tbp housekeeping gene expression. The average of the three technical replicates was considered as single data point for the statistical analysis (Supplementary Fig. 28). (D) Immunoblotting of lysed cellular fractions corroborated overexpression of either wild-type or mutant SAMD9L and the mitochondrial localization in zebrafish. DRP1 protein levels were found increased in mutants SAMD9L (N = 5) compared with either wild-type (N = 5) or control (N = 5) (Supplementary Figs 29–31). Whole-mount zebrafish immunofluorescence of WT-SAMD9L and S626L-SAMD9L showed mitochondrial staining in the zebrafish spinal cord and peripheral nerves (Supplementary Fig. 16) and in the hindbrain (E), co-localizing with the ATP5B mitochondrial marker (F) and parvalbumin (E) in WT-SAMD9L and S626L-SAMD9L zebrafish larvae neurons. Magnification bars: 20 µm. Asterisk denotes significance at P < 0.05. Vertical bars denote SEM.
|