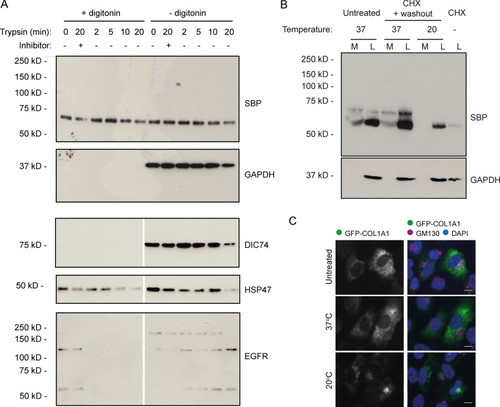
Intracellular procollagen processing in RPE1 cells. (A) Immunoblots of WT RPE1 cells stably expressing GFP-COL1A1 that were exposed to trypsin for the indicated amount of time before lysis. Lanes 2 and 8 contain lysates from cells that were treated with trypsin + an equal volume of soybean trypsin inhibitor as a negative control. Samples in lanes 1–6 were treated with digitonin for 2 min before trypsin treatment to permeabilize the cells. The DIC74, HSP47, and EGFR blots shown are all from a single membrane, but the scan images were digitally cut in half and the halves swapped to align the correct lanes with the ±digitonin labels in the figure. (B) Immunoblots of secretion assays performed on WT RPE1 cells incubated at 20°C or 37°C overnight in the presence of ascorbate. Prior to overnight incubation, cells were treated with cycloheximide (CHX) and ascorbate to flush through pre-existing procollagen. Lane 7 contains a lysate sample taken at the end of this cycloheximide treatment to show the extent to which this removed the procollagen. Lane 1 and 2 samples show media (M) and lysate (L) fractions of a cell culture treated with ascorbate only without the cycloheximide. (C) Single-plane widefield images of cells subjected to the experimental conditions of B but fixed after overnight incubation at 20°C or 37°C in the presence of ascorbate. Cells were stained by immunofluoresence for GM130 (magenta) to label the Golgi and DAPI stained (blue) to show the nuclei. Scale bars = 10 µm.
|