Fig. 1
- ID
- ZDB-FIG-200323-36
- Publication
- Gordon et al., 2019 - Fenestrae-associated protein Plvap regulates the rate of blood-borne proteins passage into the hypophysis
- Other Figures
- All Figure Page
- Back to All Figure Page
|
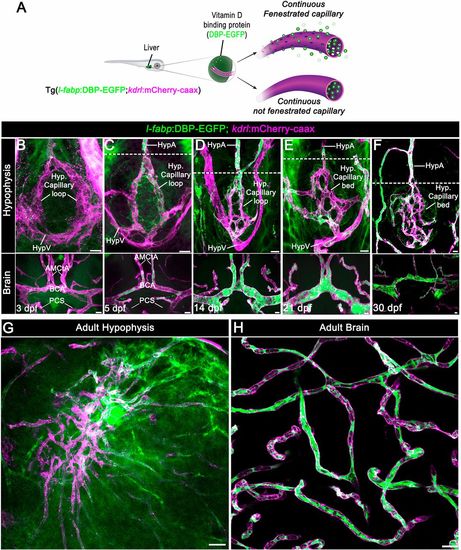
Zebrafish neurohypophyseal vasculature is permeable to blood-borne proteins. (A) Scheme describing an endogenous biosensor for real-time monitoring of vascular permeability. Vitamin D-binding protein (DBP) fused to EGFP, expressed in hepatocytes under a liver-specific promoter (l-fabp) and secreted into the general circulation, served as permeability biosensor. (B-F) Wholemounts of double transgenic Tg(l-fabp:DBP-EGFP;kdrl:mCherry-caax) zebrafish at different developmental stages demonstrating extravasation of DBP-EGFP in the pituitary (top) but not in the brain (bottom). A functional permeability boundary is established between the capillary loop and hypophyseal artery (dotted lines). Scale bars: 5 µm. (G,H) Wholemounts of double transgenic Tg(l-fabp:DBP-EGFP;kdrl:mCherry-caax) adult zebrafish hypophysis (G) and brain (H) vasculature. Scale bars: 20 µm. AMCtA, anterior (rostral) mesencephalic central artery; BCA, basal communicating artery; HypA, hypophyseal artery; HypV, hypophyseal vein; PCS, posterior (caudal) communicating segment. |