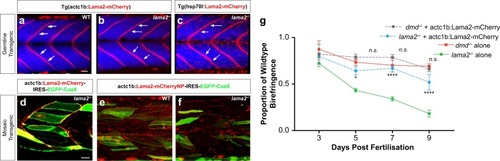
Transgenic delivery of laminin alleviates dystrophic pathology in lama2−/− animals. a, b Germline transgenic larvae expressing Tg(actc1b:Lama2-mCherry) results in correct Lama2-mCherry (red) deposition at the myosepta in WT (a) and lama2−/− deficient (b) larvae and rescues the dystrophic pathology in lama2−/− animals. c Germline transgenic lama2−/− larvae carrying the Tg(hsp70l:Lama2-mCherry) cassette are rescued upon heat-shock-induced expression of the Lama2-mCherry fusion protein (red). Blue marks differentiated muscle fibres in the Tg(actc1b:EBFP2). The focal “dot-like” Lama2-mCherry localisation results from the non-tissue-specific heat shock induction strategy, which also induces expression in non-myotomal cells. In this image, non-target expression is most prominent in cells of the epidermis. d–f Mosaic overexpression of Lama2 can rescue pathology in lama2−/− larvae. Injection of actc1b:Lama2-mCherry-IRES-GFP-CaaX into lama2−/− (d) reveals that even a low level of mosaic transgenesis (secreting transgenic cells labelled by localisation of GFP to the membranes) results in correct localisation to the myosepta, and a rescue of pathology (g). By contrast a mutant, non-polymerising (NP) form of Lama2, when expressed in the same manner (actc1b:Lama2-NPmCherry-IRES-GFP-CaaX), fails to accumulate at the myosepta in WT larvae (e) and does not rescue lama2−/− mutants (f). g Injection of actc1b:Lama2-mCherry-IRES-GFP-CaaX into dmd−/− larvae does not rescue muscle integrity. By contrast similar experiments using lama2−/− larvae results in an amelioration of muscle pathology. Results are expressed as a mean ± SD, each group n = 12 per data point. Significance is tested in a two-way ANOVA between groups at each time point; *p < 0.05, ****p < 0.0001. Scale bars, 20 μm
|

