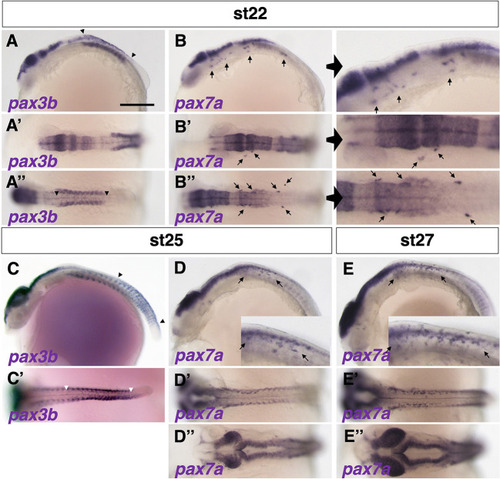
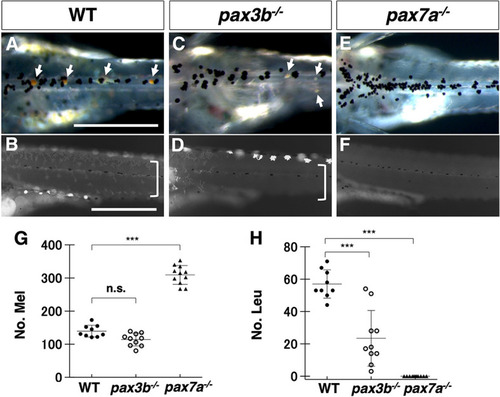
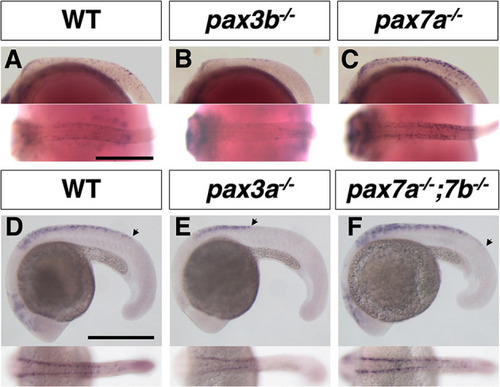
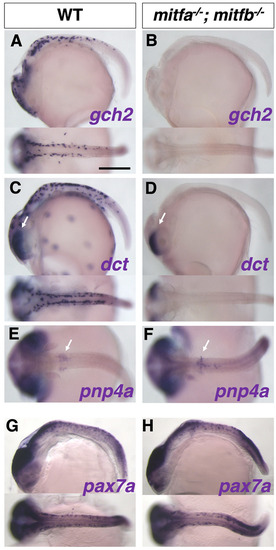
Phenotypes of medaka mitfa and mitfb double homozygotes. (A-O) Phenotypes of wild-type (WT), mitfa single, mitfb single and mitfa and mitfb double mutants in 2 dpf (A-D) and 3 dpf (E-H) embryos, 7 dpf hatchlings (I-K) and 4-month-old adult male (L,M) and female (N,O) fish. (I4,J4,K4) Autofluorescence images showing xanthophores under UV light. (A-H) Upper panels are dorsal views; lower panels are lateral views. (G-K) Panels 1, 2 and 5 are dorsal views; panels 3 and 4 are lateral views. (L-O) Lateral views. Melanophores first appear on the head, the anterior body and the yolk at 2 dpf (A) and increase in number to become distributed throughout the body at 3 dpf (E) in the WT embryo. The mitfa mutant embryo looks normal at this time (B,F), whereas the mitfb mutant does not have melanophores at 2 dpf (C), but shows their delayed formation at 3 dpf (G). The mitfa; mitfb double mutant completely lacks melanophores during this period and thereafter (D,H). At the hatching stage, all the four types of pigment cells are differentiated (pigmented) in WT (I1-I5). The mitfb mutant looks normal except that melanophores are relatively small and few leucophores are found (arrows in J2,J5) compared with those in WT (I1-I5). The mitfa; mitfb double mutant completely lacks not only melanophores (K1,K2,K5) but also xanthophores (K4) and leucophores (K1,K2,K5), but retains iridophores in the eyes and on the yolk (K1,K2,K3). Square brackets indicate xanthophores on the lateral surface of the body (I4,J4). In adulthood, compared with WT (L,M), it is obvious that the mitfa; mitfb double mutant lacks all visible pigmentation except for that of iridophores in the skin and the iris (N,O). Scale bars: 0.5 mm (in A for A-H, in I1 for I1-K1); 5 mm (in L for L-O).
|