- Title
-
Regulatory T cells regulate blastemal proliferation during zebrafish caudal fin regeneration
- Authors
- Hui, S.P., Sugimoto, K., Sheng, D.Z., Kikuchi, K.
- Source
- Full text @ Front Immunol
|
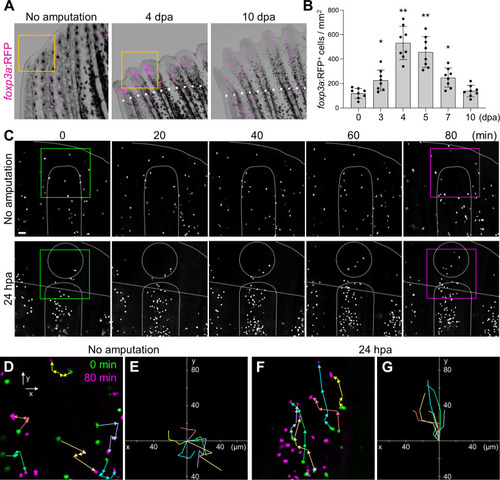
Amputation-induced infiltration of Tregs in the regenerating caudal fin tissue. (A) Spatio-temporal distribution of foxp3a:RFP+ Treg cells in the distal part of unamputated, 4 and 10 days post amputation (dpa) regenerating caudal fins. Dotted lines show the plane of amputation. Yellow box indicates the distal tip of unamputated fin or regenerating fin blastema. Bar, 200 µm. (B) Quantification of foxp3a:RFP+ cells in unamputated, 3, 4, 5, 7 and 10 dpa fins (mean ± SEM, n = 8, *P < 0.01, **P < 0.001, Mann–Whitney U test). (C) Time-lapse images of foxp3a:RFP+ cells in unamputated or 24 hours after amputation (hpa) of caudal fin. Dotted lines demarcate the distal tip, the bone, the blastema, and amputation planes. Bar, 20 µm. (D, F) Higher magnification fields of C in unamputated and 24 hpa fin respectively. foxp3a:RFP+ cells at 0 min and 80 min are indicated by green and magenta, respectively. Colored arrows correspond to the migratory tracks of each foxp3a:RFP+ cells in unamputated (D) and 24 hpa fin (F). Bar, 20 µm. (E, G) Combinatorial overlay of the 6 individual tracks of foxp3a:RFP+ cell in (D, F), respectively, and which were plotted after aligning their starting positions. Each crawling tracks display a migratory path for individual Treg cells. The traces shown in (D, F) are repositioned to the center to define the migratory path distance of each dot. Bar, 20 µm. |
|
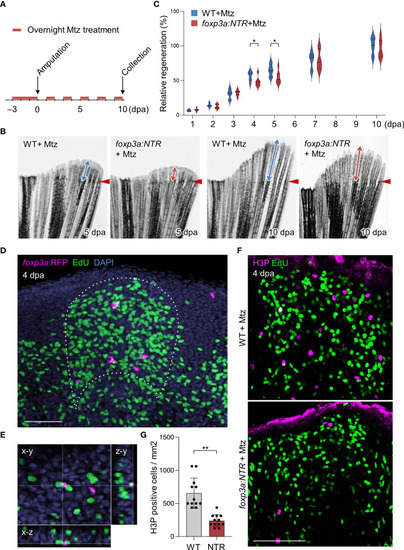
Tregs are required for blastemal proliferation during caudal fin regeneration. (A) Experimental scheme for Mtz application in foxp3a:NTR fish to achieve Treg cell-specific ablation and study caudal fin regeneration. Three continuous days of overnight treatment of Mtz were performed before the initiation of caudal fin amputation at day 0. (B) Brightfield microscopic images of caudal fins show the rate of fin regeneration after 5 and 10 dpa in wild-type and Treg cell ablated fish. (C) Rate of fin regeneration length was quantified in the wild-type fish against the Treg ablated fish. The average length of wild-type 10 dpa was considered as 100% length of fin regeneration (mean ± SEM, n = 7, Student’s T-test). (D, E) Confocal images of fin blastema at 4 dpa after EdU labeling indicates the foxp3a:RFP+ cells are spatially localized in close proximity of EdU+ blastemal cells (D) and sometimes they are also directly in contact with the EdU+ blastemal cells (E). EdU was injected intraperitoneally 30 mins before the collection of fin tissue. (F) The wholemount preparation of 4 dpa fin with EdU and H3P immunostaining in wild-type and after Treg cell ablation. (G) Quantification of H3P+ cells in the 4 dpa fin blastema of wild type and Treg ablated fish (mean ± SEM, n = 12, Mann–Whitney U test). *P < 0.01; **P < 0.001; Mtz, metronidazole; NTR, nitroreductase; Scale Bars, 50 mm. |
|
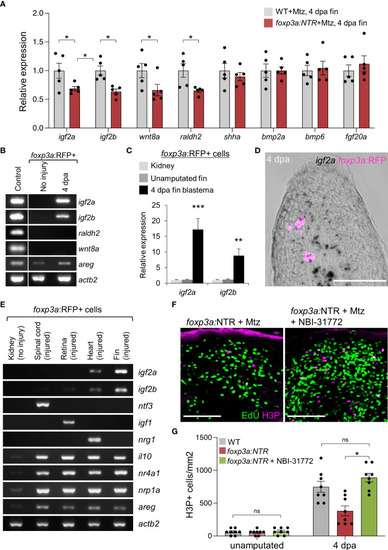
Blastemal cell proliferation during caudal fin regeneration is regulated by Treg cells-derived pro-regenerative factors. (A) qRT-PCR analysis of growth factors expression in 4 dpa fin blastema of wild-type and foxp3a:NTR fish after Mtz treatment. (mean ± SEM, n = 5, Student’s T-test). (B) RT-PCR analysis of growth factors expression (found significant decrease after Treg cell ablation) from purified foxp3a:RFP+ cells from unamputated and 4 dpa fin blastema. The 4 dpa fin blastema tissue was used as control. (C) Expression analysis of igf2a and igf2b in purified foxp3a:RFP+ cells from kidney marrow, unamputated fin and 4 dpa fin blastema. (mean ± SEM, n = 5, Student’s T-test). (D) In situ hybridization using RNAscope and immunofluorescence against TagRFP showing igf2a mRNA expression within infiltrated foxp3a:RFP+ cells of a 4 dpa fin blastema. (E) RT-PCR analysis of growth factor expression of purified foxp3a:RFP+ cells from kidney, 7 days post injured (dpi) spinal cord, 4 dpi retina, 7 dpi heart, and 4 dpa fin blastema tissues show tissue-specific growth factor secretion pattern of Treg cells. (F) The wholemount preparation of 4 dpa fin with EdU and H3P immunostaining in Treg ablated fish and fish with NBI-31772 application after Treg ablation. EdU was injected intraperitoneally at 30 min before fin tissue collection. (G) Quantification of H3P+ cells in unamputated and 4 dpa fin blastema of wild-type, Treg ablated fish, and fish with NBI-31772 application after Treg ablation (mean ± SEM, n = 8-9, Mann–Whitney U test). *P < 0.01; **P < 0.001; ***P < 0.0001; ns, not significant; Mtz, metronidazole; NTR, nitroreductase; Sacle Bars, 50 mm. |