- Title
-
Cilia regulate meiotic recombination in zebrafish
- Authors
- Xie, H., Wang, X., Jin, M., Li, L., Zhu, J., Kang, Y., Chen, Z., Sun, Y., Zha, C.
- Source
- Full text @ J. Mol. Cell Biol.
|
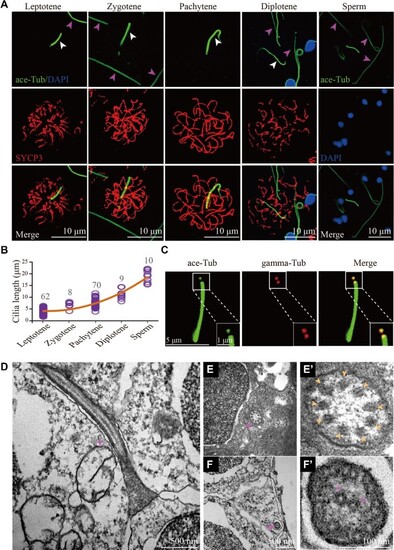
Cilia of primary spermatocytes in meiosis. (A) Confocal images showing cilia (white arrowhead) and sperm flagella (pink arrowhead) labelled with anti-acetylated tubulin (ace-Tub, green) antibody. The synaptonemal complexes were labelled with SYCP3 (red) and nuclei were counter-stained with DAPI in blue. (B) Statistical results showing the length of cilia in different stages of primary spermatocytes and sperms. (C) Confocal images showing cilia and basal bodies labelled with anti-acetylated tubulin (ace-Tub, green) and anti-gamma tubulin (gamma-Tub, red) antibodies on primary spermatocyte. (D–F’) Transmission electron microscopy results showing the ultrastructure of primary spermatocyte cilia (D–E’) and sperm flagella (F and F’). Cross section showing the ‘9 + 0’ configuration of spermatocyte cilia (E’). |
|
Generation of MZkif3a mutants via germ cell-specific expression of Cas9. (A) Bright field image showing a 24 hpf Tg(kop:cas9-p2a-egfp-UTRnanos3) zebrafish embryo with EGFP fluorescence in the PGCs. Top: diagram of the construct for making this transgene. (B) Schematic workflow showing process of generating MZ mutants using PGC-specific Cas9-expressing Tg(kop:cas9-UTRnanos3) embryos. (C) Mutation efficiencies of gametes and the phenotypes of offspring of tcf7l1a sgRNA-injected Tg(kop:cas9-UTRnanos3) fish. C1 shows the wild type-like phenotype, and C2 shows complete loss of eyes. The mutation efficiencies were calculated by the number of mutated heterozygotic embryos from crossing between F0 and wild type fish. A total of 12 embryos were genotyped from each cross and the mutation efficiency was calculated. (D) Schematic diagram showing the strategy for generating germ cell-specific knockout mutants of kif3a (gcKOkif3a). (E) External phenotypes of 48 hpf Zkif3a and MZkif3a mutants. The enlarged boxes are shown in the bottom, and dots outline the midbrain ventricles. (F and G) Statistical analysis showing the increased body curvature severity as demonstrated by the reduced angles of MZkif3a mutants. (H) Bar graph showing relative size of midbrain ventricles in different mutants as indicated. A.U., arbitrary unit. (I) Confocal images showing cilia in the spinal cord and otic vesicle of wild type, Zkif3a and MZkif3a mutants as indicated. Cilia were labelled with acetylated tubulin in green and the basal bodies were stained with gamma tubulin in red. Nuclei were counterstained with DAPI. ***P < 0.001; ****P < 0.0001. ns, not significant; hpf, hours post-fertilization; wt, wild type. |
|
Phenotypes of germ cell-specific kif3a conditional knockout mutants. (A) External phenotypes of wild type and Tg(kop:cas9-UTRnanos3;U6:3×sgRNA-kif3a) double transgenic male fish. (B) The embryos produced from wild type female crossed with control or double transgenic male at 6 hpf. (C) Statistical results showing the percentages of fertilization rates of wild type and double transgenic males as indicated. (D) Histological analysis of testis of wild type and Tg(kop:cas9-UTRnanos;U6:3×sgRNA-kif3a) double transgenic fish. Arrowheads point to spermatocytes and asterisks indicate spermatozoa. (E) Confocal images showing sperm flagella in wild type and double transgenic fish labelled with anti-acetylated tubulin antibody (green). (F) Statistical analysis of the length of sperm flagella in wild type and double transgenic fish. (G) Confocal images showing cilia in primary spermatocytes of wild type and double transgenic fish. Spermatocyte cilia (green) were absent in the double transgenic fish. The stages of spermatocytes were distinguished by the staining of SYCP3 (red). (H) Staining of SYCP3 (red) and acetylated-tubulin (green) in the testis of wild type and double transgenic fish. Arrows indicate spermatocyte cilia, while arrowheads indicate the staining of abnormal sperm flagella in the mutant testis. ****P < 0.0001. wt, wild type. |
|
The repair of DSBs is compromised in the absence of spermatocyte cilia. (A) Confocal images showing the staining of γ-H2A.X (red) in the testis of wild type and Tg(kop:cas9-UTRnanos3;U6:3×sgRNA-kif3a) double transgenic fish. (B) Statistical analysis showing relative fluorescence intensity of γ-H2A.X staining on leptotene and zygotene spermatocytes of wild type and double transgenic fish. (C) Statistical analysis of the number of spermatocytes per spermatocyst in wild type and double transgenic fish. (D) Staining of Rad51 in the testis of wild type and double transgenic fish. (E) Statistical analysis of relative fluorescence intensity of Rad51 staining on leptotene and zygotene spermatocytes. (F) Staining of Mlh1 in primary spermatocytes of wild type and double transgenic fish. (G) Statistical analysis of the Mlh1 foci number per spermatocyte. (H and I) Apoptotic cells stained by TUNEL assay in wild type and double transgenic fish. *P < 0.05; ***P < 0.001; ****P < 0.0001. ns, not significant; wt, wild type. |
|
Ift88 deficiency results in ciliogenesis defects and apoptosis of spermatocytes. (A) Schematic workflow showing the strategy of generating Tg(hsp70l:ift88-egfp) transgene to rescue ovl(ift88) mutants. (B) Statistical analysis of the percentage of ciliated spermatocytes in heat-shocked and non-heat-shocked ift88;Tg(hsp70l:ift88-egfp) transgenic fish. (C) Confocal images showing cilia and synaptonemal complexes labelled with anti-acetylated tubulin (green) and anti-SYCP3 (red) antibodies on primary spermatocytes from different fish as indicated. (D and E) Confocal images showing apoptotic cells stained by TUNEL assay (D) and the statistical results (E) of TUNEL-positive cells in heat-shocked and non-heat-shocked fish as indicated. **P < 0.01. wt, wild type. |
|
Model of spermatocyte cilia during meiosis. During the prophase of meiosis I, cilia are present in the spermatocyte from leptotene to pachytene stages. This cilium participates in the regulation of HR. In the absence of cilia, less Rad51 proteins are recruited to the DSBs, which compromises the efficiency of DSB repair, causing accumulation of γ-H2A.X signals and decreased level of crossover formation. wt, wild type. |