- Title
-
A membrane arm of mitochondrial complex I sufficient to promote respirasome formation
- Authors
- Fang, H., Ye, X., Xie, J., Li, Y., Li, H., Bao, X., Yang, Y., Lin, Z., Jia, M., Han, Q., Zhu, J., Li, X., Zhao, Q., Yang, Y., Lyu, J.
- Source
- Full text @ Cell Rep.
|
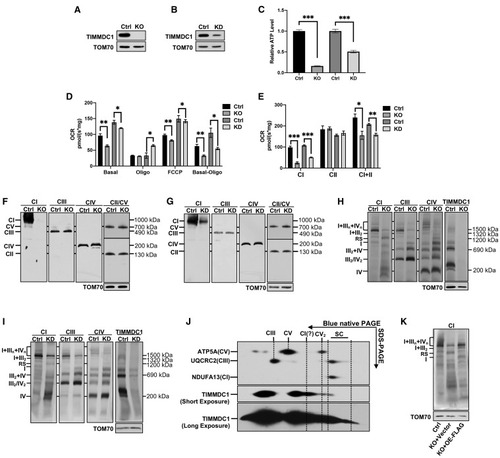
Figure 1. Loss of TIMMDC1 leads to the accumulation of respirasome subcomplex (A and B) Western blot (WB) analysis of the steady-state levels of TIMMDC1 in HEK293T cells with KO of TIMMDC1 (A), KD of TIMMDC1 (B), and paired control cells (Ctrl). TOM70 was used as an internal control. (C) Cellular ATP content in HEK293T cells with KO and KD of TIMMDC1 and paired control cells (Ctrl). (n = 3) (D) Cell respiration was determined by measuring the oxygen consumption rate (OCR) in HEK293T cells with KO and KD of TIMMDC1 and in paired control cells (Ctrl). Endogenous respiration is presented as follows: basal, basal-cell respiration; oligo, the non-phosphorylating proton leaked respiration measured in the presence of oligomycin (2 μg/mL); FCCP, un-coupling respiration measured in the presence of carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (2.5 μM); basal-oligo, absolute respiration of ADP to ATP phosphorylation. (n = 3) (E) Respiratory complex-dependent OCR in HEK293T cells with KO and KD of TIMMDC1 and in paired control cells (Ctrl). CI, respiration in the presence of malate and glutamate. CII, respiration in the presence of succinate. CI+CII, respiration in the presence of malate, glutamate, and succinate. (n = 3) (F and G) Steady-state levels of OXPHOS complexes were analyzed in HEK293T cells with KO (F) and KD (G) of TIMMDC1 and in paired control cells (Ctrl). N-dodecyl β-d-maltoside (DDM) solubilized protein was separated by 3.5%–16% blue-native PAGE. OXPHOS complexes were detected by immunoblotting with antibodies against proteins as indicated (CI, NDUFA13; CII, SDHA; CIII, UQCRC2; CIV, MT-CO1; CV, ATP5A). Complexes I–V are abbreviated as CI–V. TOM70 was used as an internal control. (H and I) Respiratory supercomplexes were analyzed in HEK293T cells with KO (H) and KD (I) of TIMMDC1 and in paired control cells (Ctrl). Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE. Respiratory supercomplexes were immunoblotted with antibodies as indicated (CI, NDUFB6; CIII, UQCRC2; CIV, MT-CO1). TOM70 was used as an internal control. (J) 2D blue-native PAGE/tricine SDS-PAGE and immunoblotting analysis of HEK293T cells. Digitonin-solubilized protein was separated by 1D 3%–11% blue-native PAGE, followed by 2D tricine SDS-PAGE. OXPHOS complexes were immunoblotted with anti-CI (NDUFB6), -CIII (UQCRC2), -CV (ATP5A), and -TIMMDC1 antibodies. (K) Blue-native PAGE and immunoblotting analysis of HEK293T cells (Ctrl), TIMMDC1−/− cells with empty vector (KO+vector), and TIMMDC1−/− cells with re-expression of FLAG-tagged TIMMDC1 (KO+OE-FLAG; OE [overexpression]). Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE and then immunoblotted with the anti-NDUFB6 (CI) antibody. TOM70 was used as an internal control. Quantitative data were generated from three independent experiments. Data are presented as the means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. |
|
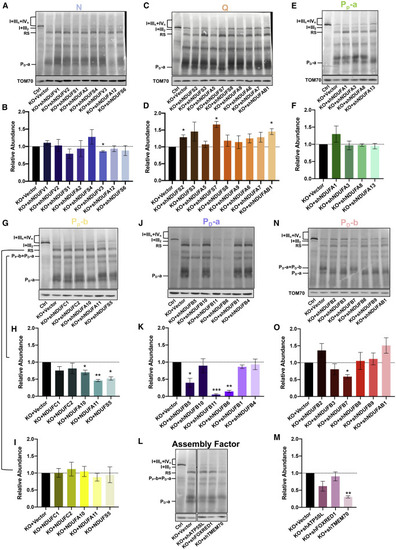
Figure 2. Respirasome subcomplex contains an immature complex I (A) Cartoon of complex I with different modules. The model was prepared based on modifications to the existing model (Guerrero-Castillo et al., 2017). Subunits named in the cartoon are shown in (B) and (C). Subunits of each module are shown in the figure table (bottom). (B and C) Blue-native PAGE and immunoblotting analysis of N, Q, PP-a, PP-b, PD-a, and PD-b modules and assembly factors in the respirasome subcomplex (RS). Representative subunits corresponding to N, Q, PP-a, PP-b, PD-a, and PD-b modules and assembly factors were immunoblotted in digitonin-solubilized proteins separated by 3%–11% blue-native PAGE. N, Q, PP-a, PP-b, PD-a, and PD-b modules and assembly factors were immunoblotted with anti-NDUFS1 and NDUFS4 (B), anti-NDUFS3 and NDUFA9 (B), anti-NDUFA13 (B), anti-NDUFC2 and NDUFA11 (C), anti-NDUFB1, -NDUFB6, and -NDUFB11 (C), and anti-NDUFAF1, -FOXRED1 (B), and anti-ACAD9 (C), respectively. TOM70 was used as internal control. Ctrl, HEK293T cells; KO, TIMMDC1−/− cells. (D) In-gel activity assay (IGA) for CI and CIV was performed in control (Ctrl) and TIMMDC1−/− (KO) cells. Digitonin-solubilized protein was separated by 3%–13% blue-native PAGE and incubated with substrates for the detection of CI, CIV, and CI+CIV. CI was immunoblotted with anti-NDUFB6 antibody. Coomassie-blue-stained blue-native gel (blue) was used as a loading control. |
|
Figure 3. The PD-a module of complex I is a structural component of the respirasome subcomplex (A–I) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with sequence-specific KD of nuclear-encoded, constitutive subunits of CI. Eight subunits of N (A), nine subunits of Q (C), four subunits of PP-a (E), and five subunits of PP-b (G) were knocked down in a total of 26 TIMMDC1−/− cell lines. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (A, C, E, and G). Quantitative analysis of RS levels was performed with KD of N (B), Q (D), PP-a (F), and PP-b (I) modules. PP-b+PD-a-containing complex in TIMMDC1−/− cells with KD of PP-b module (G) was quantified as in (H). (J and K) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with sequence-specific KD of nuclear encoded constitutive subunits of PD-a modules. Six subunits of the PD-a modules were knocked down independently in six TIMMDC1−/− cells. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (J). Quantitative analysis of RS levels was performed with KD of PD-a (K). (L and M) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with sequence-specific KD of assembly factors. ATP5SL, FOXRED1, and TMEM70 were knocked down independently in three TIMMDC1−/− cells. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (L). Quantitative analysis of the RS levels was performed with the KD of ATP5SL, FOXRED1, and TMEM70 (M). (N and O) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with sequence-specific KD of nuclear-encoded constitutive subunits of PD-b modules. Six subunits of the PD-b modules were knocked down independently in TIMMDC1−/− cells. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (N). Quantitative analysis of RS levels was performed with KD of PD-b (O). TOM70 was used as an internal control. Quantitative data were generated from three independent experiments. Data are presented as the means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. |
|
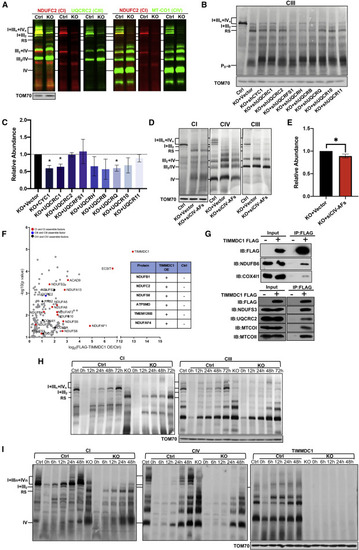
Figure 4. Respirasome subcomplex contains CIII/CIV for TIMMDC1-mediated respirasome assembly (A) Blue-native PAGE and fluorescent immunoblotting analysis of respiratory supercomplexes in control (Ctrl) and TIMMDC1−/− (KO) cells. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE and immunoblotting with anti-NDUFC2 (CI), -UQCRC2 (CIII), -MT-CO1 (CIV), and fluorophore-conjugated secondary antibodies. (B and C) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with sequence-specific KD of nuclear-encoded constitutive subunits of CIII. Nine nuclear-encoded subunits for CIII were knocked down independently in TIMMDC1−/− cells. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (B). Quantitative analysis of RS levels was performed with KD of CIII (C). (D and E) Blue-native PAGE and immunoblotting analysis of the RS in control (Ctrl) and TIMMDC1−/− (KO) cells with small interfering (siRNA)-mediated KD of four assembly factors (COX14, COA1, COA3, and SURF1) of CIV. TIMMDC1−/− cells were transfected with four siRNA sequences simultaneously. Digitonin-solubilized protein was separated by 3%–11% blue-native PAGE, and the RS was immunoblotted with anti-NDUFB6 antibody (D). Quantitative analysis of RS levels was performed (E). (F) Protein interactions with exogenous TIMMDC1 were detected by co-immunoprecipitation/liquid chromatography-mass spectrometry (co-IP/LC-MS) in HEK293T cells. Proteins were sorted by fold-change and p value (<0.05). Proteins that were not found in control cells but were abundant in TIMMDC1 OE cells and, therefore, have no fold-change or p value, are included in the figure table (right panel). (G) Protein interactions with exogenous TIMMDC1 were detected by coIP/WB in HEK293T cells. The exogenous TIMMDC1 was tagged with FLAG, and anti-FLAG antibody was used to immunoprecipitate the protein complex. Antibodies against CI (NDUFB6 and NDUFS3), CIII (UQCRC2), and CIV (MTCO1, MTCO2, and COX4I1) were used to detect respiratory complexes co-immunoprecipitating with TIMMDC1. (H and I) Assembly dynamics of respirasome and the RS in TIMMDC1−/− cells and paired control (Ctrl) cells. Cells were pretreated with chloramphenicol (CAP, 40 μg/mL) for 7 days. Assembly of respirasome, RS, and TIMMDC1 in digitonin-solubilized cells was examined by CAP removal, followed by blue-native PAGE (3%–11%). Respirasome and the RS were identified by antibodies against CI (NDUFB6) and CIII (UQCRC2) (H) or CI (NDUFB6), CIV (MT-CO1), and TIMMDC1 (I). TOM70 was used as an internal control. Quantitative data were generated from three independent experiments. Data are presented as the means ± SEM. ∗p < 0.05. |
|
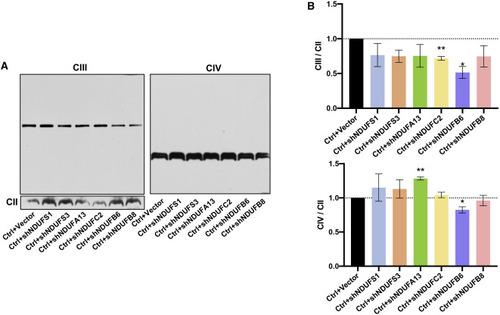
Figure 5. Respirasome subcomplex preserves the steady-state levels of holo-CIII and CIV Blue-native PAGE and immunoblotting analysis of the CIII and CIV (A) in HEK293T cells with sequence-specific KD of six subunits from each module and control HEK293T cells (Ctrl). DDM-solubilized protein was separated by 3.5%–16% blue-native PAGE. CII (SDHA) was used as an internal control. Quantitative analysis of CIII and CIV (B) was performed with three independent experiments and presented as the means ± SEM. ∗p < 0.05, ∗∗p < 0.01. |
|
Figure 6. TIMMDC1 deficiency led to the accumulation of respirasome subcomplex in a patient sample (A) Western blot analysis of the steady-state levels of TIMMDC1 in immortalized lymphocytes derived from patient (P) and asymptomatic mother (C). The level of TOM70 was used as an internal control (n = 3). (B) Cellular ATP content in immortalized lymphocytes derived from patient (P) and his asymptomatic mother (C) was normalized to protein concentration (n = 3). (C) Cell respiration was determined by measuring OCR in immortalized lymphocytes derived from patient (P) and his asymptomatic mother (C). Endogenous respiration was determined as follows: basal, basal cell respiration; oligo, the non-phosphorylating proton-leak respiration measured in the presence of oligomycin (2 μg/mL); FCCP, uncoupling respiration measured in the presence of FCCP (2.5 μM); basal-oligo, absolute respiration of ADP to ATP phosphorylation (n = 3). (D) Blue-native PAGE (3.5%–16%) analysis of OXPHOS complexes using DDM-solubilized cell lysate in immortalized lymphocytes derived from patient (P) and his asymptomatic mother (C). Antibodies against NDUFA13, SDHA, UQCRC2, COX I, and ATP5A were used to detect complex I (CI), complex II (CII), complex III (CIII), complex IV (CIV), and complex V (CV), respectively. TOM70 was used as an internal control. (E) PCR analysis of timmdc1 KD in zebrafish. About 500-bp PCR product: aberrant splicing of timmdc1 mRNA after being treated with MOs; 115-bp PCR product, wild-type splicing of timmdc1 mRNA in exons 2 and 3. Actin was used as an internal control. WT, wild-type zebrafish; Ctrl, zebrafish with injection of control-MOs; MO-0.25/0.5/0.75/1, zebrafish treated with different concentrations of MOs. (F) Blue-native PAGE analysis of complex I in control (Ctrl) and timmdc1 KD (MO) zebrafish with anti-NDUFS3 antibodies. KD of timmdc1 in zebrafish was generated by treating the embryos with MOs for 3 days. VDAC was used as an internal control. (G) The development of zebrafish embryos after injection of MOs was observed at 0, 24, 48, and 96 h. Thoracic cysts were observed in the timmdc1-MOs group. Ctrl, control zebrafish (H) Touch response of control (Ctrl) and timmdc1 KD (MO) zebrafish at 48 hours post-fertilization (hpf; n = 24). (I) Spontaneous movements of control (Ctrl) and timmdc1 KD (MO) zebrafish embryos at 19–24 hpf (n = 80). (J and K) Blue-native PAGE (3%–11%) analysis of supercomplexes using digitonin-solubilized protein in immortalized lymphocytes derived from patient (P) and his asymptomatic mother (C). Antibodies against NDUFB6 (for CI), UQCRC2 (for CIII), MT-CO1 (for CIV), and TIMMDC1 were used for detection of supercomplexes (J). CI modules were analyzed by immunoblotting with antibodies against the N module: NDUFS1 and NDUFS4 (J); Q module: NDUFS3 and NDUFA9 (J); PP-a module: NDUFA13 (J); PP-b module: NDUFC2 and NDUFA11 (K); PD-a module: NDUFB1, NDUFB6, and NDUFB11 (K); and PD-b module: NDUFB7, NDUFB8, and NDUFB9 (K). TOM70 was used as an internal control. MO, morpholino oligonucleotides; quantitative data were generated from at least three independent experiments. Data are presented as the means ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001. EXPRESSION / LABELING:
|