- Title
-
The Heparan Sulfate Binding Peptide in Tumor Progression of Triple-Negative Breast Cancer
- Authors
- Melo, C.M., Wang, H., Fujimura, K., Strnadel, J., Meneghetti, M.C.Z., Nader, H.B., Klemke, R.L., Pinhal, M.A.S.
- Source
- Full text @ Front Oncol
|
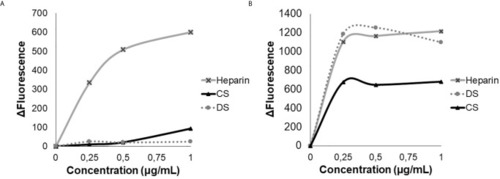
Analysis of tryptophan emission. Heparin, dermatan sulfate (DS) and chondroitin sulfate (CS), were titrated in the presence of 3 µM of HS-binding peptide |
|
A spectrum obtained by circular dichroism. Circular dichroism was performed to determine the peptide interaction with chemically modified heparins, demonstrating an alteration in the secondary structure of this peptide. Porcine heparin (Hep), Heparin 2-O-sulfated and N-sulfated heparin (Hep2SNS), Heparin 6-O-sulfated and N-sulfated (Hep6SNS), Heparin 2-O-sulfated and 6-O-sulfated (Hep2S6S), Heparin 6-sulfated (Hep6S), Heparin 2-sulfated (Hep2S), and Heparin N-sulfated (HepNS). The heparins that showed the greatest interaction with the peptide were the molecules containing N-sulfation. The assay was performed in the presence of 12 µM of the HS binding peptide and 6.7 µg/ml of each heparin in a 10 mM sodium phosphate solution. Control, 12µM of peptide in 10mM sodium phosphate solution. |
|
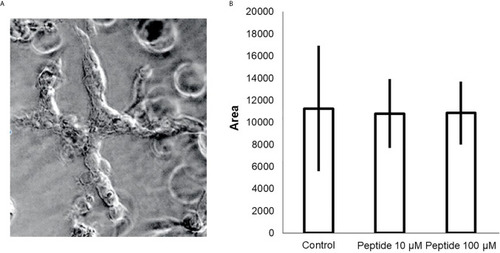
Angiogenesis |
|
Analysis of angiogenesis in |
|
|
|
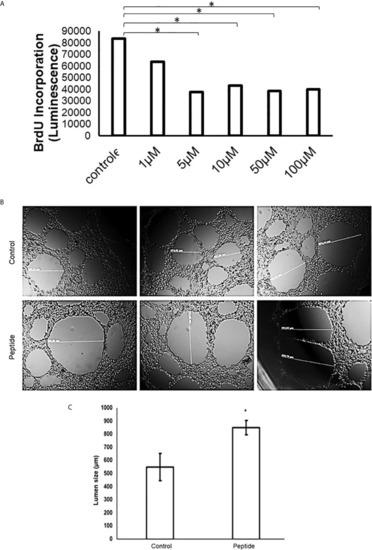
Cell proliferation/viability assay in the presence of growth factors. HUVEC cells were cultured for 50 hours on collagen type 1 in medium containing 2% fetal bovine serum (FBS) and 20 ng/mL FGF-2 or 50 ng/mL VEGF-A, in the presence or absence of the HS-binding peptide. |
|
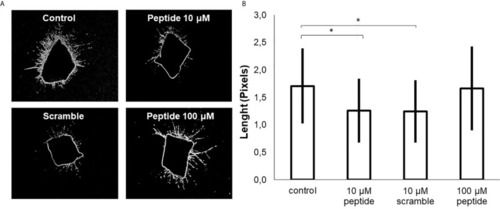
Effect of HS-binding peptide in the 3D culture (spheroid) triple-negative breast cancer. Patient-derived xenotransplant cells (PDX cells) were obtained from tissue collected of a triple negative breast cancer patient. |
|
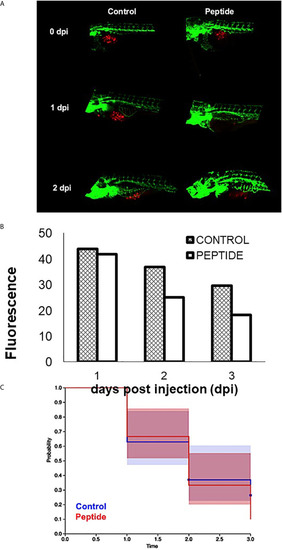
Effect of HS-binding peptide on tumor progression. Approximately 150 PDX cells obtained by surgical resection of a patient with triple-negative breast cancer were labeled with red fluorescent protein (RFP) and injected into the zebrafish embryo yolk sac after 1 day of fertilization (1 dpf). Embryos were incubated for 18 hours, 35.5°C. Peptide, animals were treated with 10 µM HS-binding peptide. Control, animals were not treated. Green; green fluorescent protein (GFP) labeled blood vessels. Red; red fluorescent protein (RFP) labeled tumor cells. |