- Title
-
In Vivo Characterization of Endogenous Cardiovascular Extracellular Vesicles in Larval and Adult Zebrafish
- Authors
- Scott, A., Sueiro Ballesteros, L., Bradshaw, M., Tsuji, C., Power, A., Lorriman, J., Love, J., Paul, D., Herman, A., Emanueli, C., Richardson, R.J.
- Source
- Full text @ Arterio., Thromb., and Vas. Bio.
|
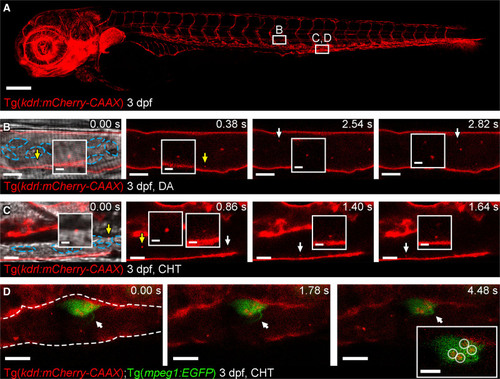
Figure 1. Cell-type specific extracellular vesicle (EV) labeling strategy and live imaging in the peripheral circulation.A, Overview image of a Tg(kdrl:mCherry-CAAX) fish at 3 dpf. All endothelial cells are labeled with mCherry. The boxed areas define the regions shown in the image sequence in B, C, D. B, Image sequence of mCherry+ endothelial cell (EC)-EVs moving through the dorsal aorta (DA; arrows and inset). The resolution of light-based detection methods limits our ability to accurately determine the size of endogenous EVs during live imaging. C, Image sequence of mCherry+ EC-EVs moving through the caudal hematopoietic tissue (CHT; arrows and inset). D, Image sequence of a macrophage (green) in the CHT of a Tg(kdrl:mCherry-CAAX);Tg(mpeg:EGFP) fish at 3 dpf, a macrophage protrusion moves toward the cell body (arrows) and intracellular compartments contain mCherry+ EC-EVs (circled in inset). The blue dashed lines in B, C demark blood cells. The white dashed line in D demarks the endothelium. Anterior is to the left. Scale bars: A, 200 μm; B and C, 5 μm; insets in C, 2 μm; D, 10 μm; insets in D, 5 μm. |
|
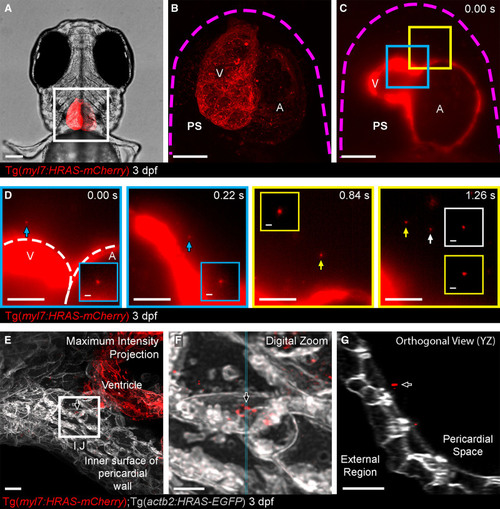
Figure 2. Cell-type specific extracellular vesicle (EV) labeling strategy and live imaging in the pericardial space (PS).A, Ventral view of 3 dpf Tg(myl7:HRAS-mCherry) zebrafish, boxed region highlights position of B and C. B and C, Overview images of the entire hearts of Tg(myl7:HRAS-mCherry) fish in ventral view. B, A maximum projection of a fixed fish; (C) a single plane of live light sheet imaging. D, Image sequences of higher magnification views of the color coded boxed areas in C. mCherry+ CM-EVs are observed moving through the pericardial space (arrowed and inset). E–G, A maximum intensity projection of deconvolved images of the ventricle and internal surface of the pericardial wall (E) reveals static CM-EVs as shown with digital zoom of boxed region (F). The orthogonal view (YZ) of this region suggests the EVs may be associated with a layer of unmarked ECM (extracellular matrix) rather than direct contact with underlying cells (G). The magenta dashed line in B and C demarks the pericardial wall. The white dashed line in D demarks the ventricle (V) and atrium (A). Arrows indicate static EVs. Anterior is to the left. Scale bars: A, 100 μm; B and C, 50 μm; D, 20 μm; insets in D, 2 μm; E and G, 5 μm; F, 2 μm. |
|
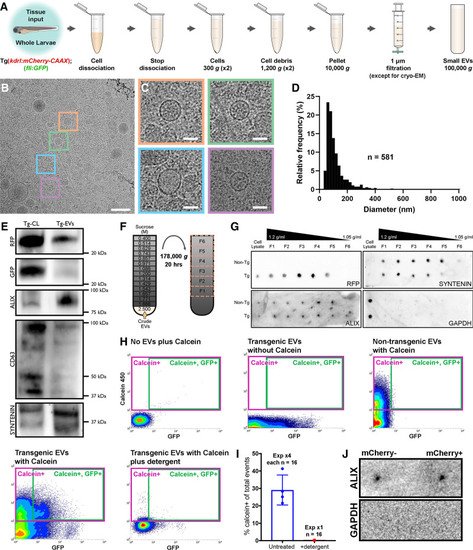
Figure 3. Validation of endogenous cardiovascular extracellular vesicles (EVs) from larval zebrafish.A, Schematic describing the centrifugation steps taken to isolate EV fractions following cell dissociation of whole zebrafish larvae. B and C, Cryo-EM micrograph of isolated EVs from a pool of whole 3 dpf larvae (n=1200). C, A panel of 4 higher magnification views of the boxed regions in B. D, Histogram of the size distribution of EVs visualized by cryo-EM. n refers to number of EVs analyzed. E, Western blot analysis of protein extracted from the cells (Tg-CL) and crude EV fraction (Tg-EVs) isolated from Tg(kdrl:mCherry-CAAX); Tg(fli:GFP) 6 dpf larvae (n=600). F, Schematic describing the sucrose gradient approach used to more precisely isolate EVs from the crude EV fraction. G, Dot blot analysis of protein extracted from the cells and sucrose gradient fractions isolated from nontransgenic (Non-Tg [n=600]) and Tg(kdrl:mCherry-CAAX) (Tg [n=600]) 6 dpf larvae. H, Typical flow cytometry scatter plots showing the gates used to sort EVs and the controls used to define these gates: An extraction buffer only control plus calcein AM reveals background noise, EVs extracted from Tg(actb2:HRAS-EGFP) fish without calcein AM indicates GFP+ EVs, EVs extracted from nontransgenic (wildtype) fish and labeled with calcein AM defines a calcein+ gate and analysis of EVs extracted from Tg(actb2:HRAS-EGFP) fish with calcein AM labeling identifies a gate of GFP+ calcein+ EVs. Treatment of transgenic EVs labeled with calcein AM plus detergent destroys the majority of EVs, confirming their lipidaceous structure. Similar gating strategies can be used to analyze mCherry+ EVs from Tg(kdrl:mCherry-CAAX) and Tg(myl7:HRAS-mCherry) fish. I, Plot of the number of calcein+ EVs of total events from untreated and detergent treated samples. J, Dot blot analysis of protein extracted from FAVS isolated particles, sorted for both mCherry- and mCherry+ EVs from Tg(kdrl:mCherry-CAAX) 6 dpf larvae, confirms expression of the EV component Alix and absence of Gapdh expression. Scale bars: B, 200 nm; C, 50 nm. |
|
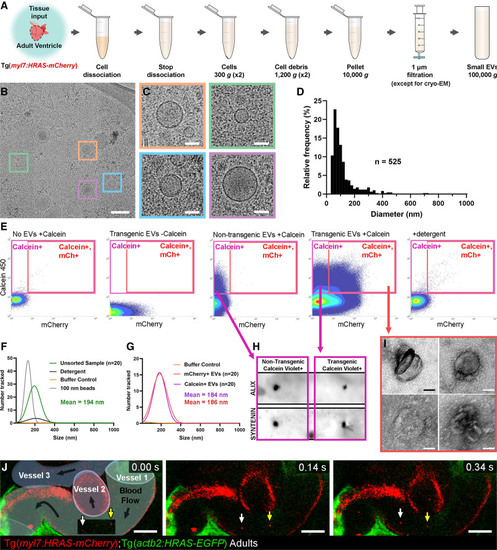
Figure 4. Validation of endogenous cardiovascular extracellular vesicles (EVs) from adult zebrafish.A, Schematic describing the centrifugation steps taken to isolate EV fractions following cell dissociation of adult zebrafish ventricular tissue. B and C, Cryo-EM micrograph of isolated EVs from a pool of ventricles (n=60). C, A panel of 4 higher magnification views of the boxed regions in B. D, Histogram of the size distribution of EVs visualized by cryo-EM. n refers to number of EVs analyzed. E, Typical flow cytometry scatter plots showing the gates used to sort adult cardiac EVs and the controls used to define these gates. F and G, Gaussian distribution of NTA analysis on unsorted EV fractions before and after detergent treatment (F) and after sorting for calcein+ EVs and mCherry+ calcein+ EVs (G). H, Dot blot analysis of protein extracted from FAVS isolated particles, sorted for calcein+ EVs from both nontransgenic and Tg(myl7:HRAS-mCherry) adult ventricular tissue, confirms expression of the EV components Alix and Syntenin. I, TEM-negative stain micrographs of FAVS isolated particles, sorted for mCherry+ calcein+ EVs from Tg(myl7:HRAS-mCherry) adult ventricular tissue. J, Schematic overlay describing the position of the 3 vessels visible in the integrated time series of live imaging of endothelial cell-EVs in the peripheral circulation of an adult Tg(actb2:HRAS-EGFP); Tg(kdrl:mCherry-CAAX) double transgenic fish. White and yellow arrows indicate 2 EC-EVs moving with the blood flow. Scale bars: B, 200 nm; C and I, 50 nm; J, 10 μm. |
|
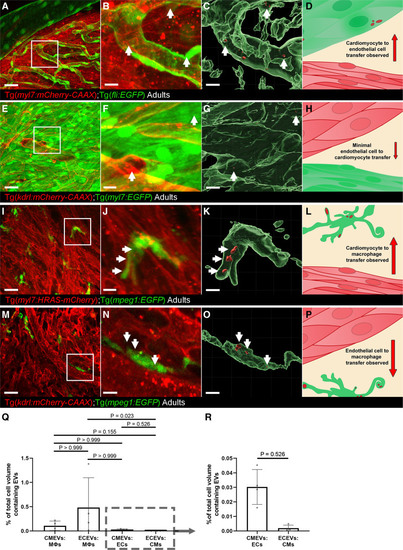
Figure 5. Whole-mount high-resolution imaging of fixed adult hearts to characterize extracellular vesicle (EV) transfer to recipient cells.A–D, Cardiomyocyte (CM)-EV transfer to endothelial cells (ECs). A, Overview maximum projection showing the surface view of an adult Tg(myl7:HRAS-mCherry); Tg(fli:EGFP) zebrafish ventricle, boxed region highlights position of B and C. B and C, Digital zoom of the boxed region in A. B, A maximum projection; C, Three-dimensional reconstruction of the same image reveal CM-EVs within ECs (CMs removed for clarity). D, Schematic summary depicts localization of CM-EVs within ECs. E–H, Limited EC-EV transfer to CMs. E, Overview maximum projection showing the surface view of an adult Tg(kdrl:mCherry-CAAX); Tg(myl7:EGFP) zebrafish ventricle, boxed region highlights position of F and G. F and G, Digital zoom of the boxed region in E. F, A maximum projection; G, A 3-dimensional reconstruction of the same image reveal EC-EVs within CMs (ECs removed for clarity). H, Schematic summary depicts localization of EC-EVs within CMs. I–L, CM-EV transfer to macrophages. I, Overview maximum projection showing the surface view of an adult Tg(myl7:HRAS-mCherry); Tg(mpeg:EGFP) zebrafish ventricle, boxed region highlights position of J and K. J and K, Digital zoom of the boxed region in I. J, A maximum projection; K, A 3-dimensional reconstruction of the same image reveal CM-EVs within macrophages (CMs removed for clarity). L, Schematic summary depicts localization of CM-EVs within macrophages. M–P, EC-EV transfer to macrophages. M, Overview maximum projection showing the surface view of an adult Tg(kdrl:mCherry-CAAX); Tg(mpeg:EGFP) zebrafish ventricle, boxed region highlights position of N and O. N and O, Digital zoom of the boxed region in M. N, A maximum projection; O, A 3-D reconstruction of the same image reveal EC-EVs within macrophages (ECs removed for clarity). P, Schematic summary depicts localization of EC-EVs within macrophages. Q and R, Volume measurements of EV containing compartments are shown as a percentage of volume measurements of the cells within the field of view from 3D image analysis. The boxed region in Q highlights the expanded view in R. Arrows indicate EVs within recipient cells. Scale bars: A, E, I, M=20 μm; B, C, F, G, J, K, N, O=5 μm. |
|
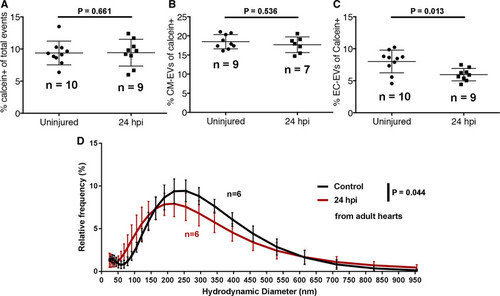
Figure 6. A model of myocardial infarction (MI) induces dynamic changes in cardiovascular extracellular vesicles (EVs) from adult zebrafish. A–C, Quantification of the number of calcein+ (A), myl7(mCherry)+ (B), and kdrl(mCherry)+ EVs in uninjured and injured hearts at 24 hpi. D, Histogram of DLS analysis reveals a significant shift in the size of overall EVs at 24 hpi compared with uninjured hearts. Statistical analysis in A–C, 2-tailed Mann-Whitney U tests. Statistical analysis in D: a custom permutation test using total variation distance was used to test the null hypothesis that control and 24 hpi distributions were the same. |