- Title
-
Distribution and Restoration of Serotonin-Immunoreactive Paraneuronal Cells During Caudal Fin Regeneration in Zebrafish
- Authors
- König, D., Dagenais, P., Senk, A., Djonov, V., Aegerter, C.M., Jaźwińska, A.
- Source
- Full text @ Front. Mol. Neurosci.
|
Schematic representation of an adult zebrafish caudal fin. |
|
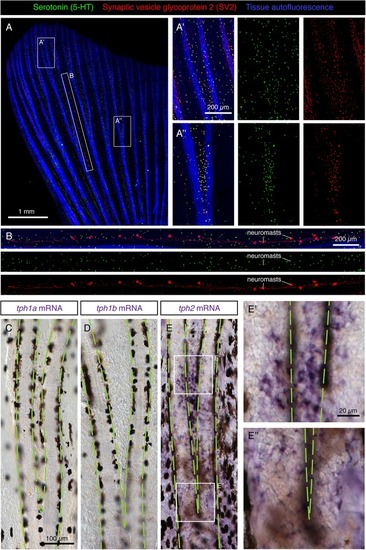
Molecular markers of HCS-cells in the adult fin epidermis. |
|
Characterization of HCS-cells in the adult uninjured fin. |
|
Electron microscopy images of the fins reveal small round cells in the subsuperficial layer of the epidermis. |
|
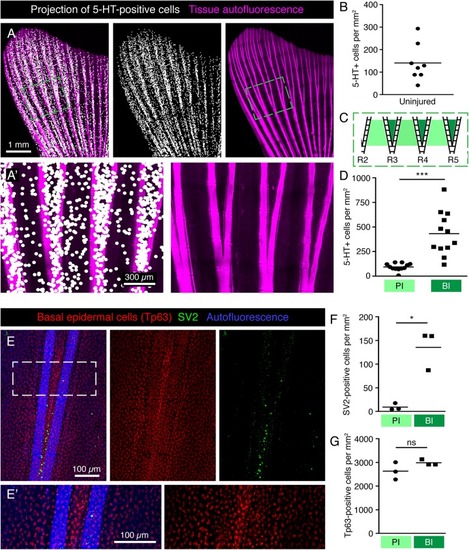
Scattered distribution of HCS-cells in uninjured adult fins. |
|
Specific pattern of HCS-cells around bifurcations in the uninjured fin. |
|
DASPEI-positive cells in the uninjured fin do not display a HCS-like distribution pattern. |
|
Distribution of HCS-cells in |
|
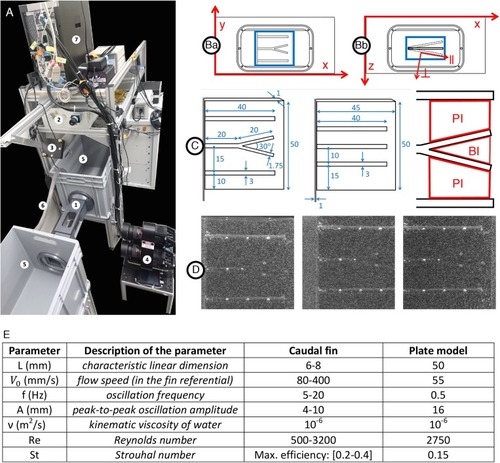
Experimental setup for hydrodynamics measurement. |
|
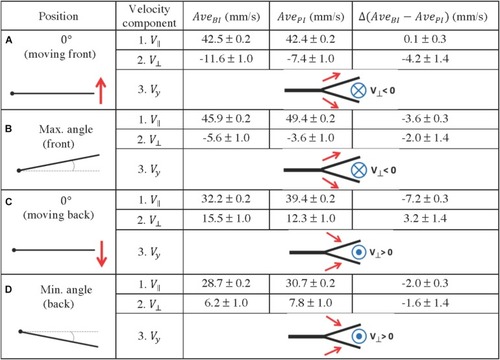
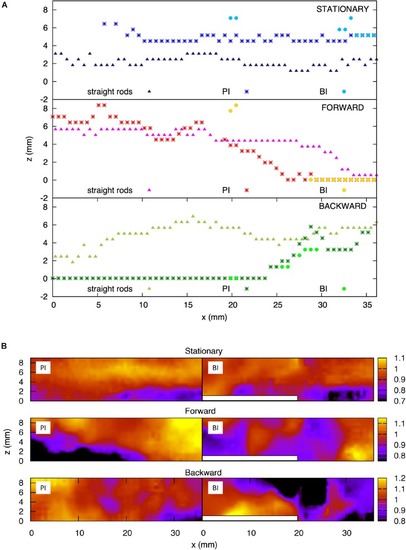
Fluid velocity profiles in the bifurcation interray zone (BI) and the primary interray zone (PI) highlight particular fluid motion at the interray bifurcation site. |
|
Quantification of fluid profiles in the bifurcation interray zone (BI) and the primary interray zone (PI), at four equidistant positions of the fin model during a complete period. |
|
Analysis of the boundary layer on scaled fin models. |
|
Restoration of HCS-cells during fin regeneration after amputation. |
|
Low proliferative rate of mature HCS-cells during fin regeneration. |
|
Inhibition of Serotonin production by pcpa-treatment does not prevent HCS-cell regeneration. |