- Title
-
Low temperature mitigates cardia bifida in zebrafish embryos
- Authors
- Lin, C.Y., Huang, C.C., Wang, W.D., Hsiao, C.D., Cheng, C.F., Wu, Y.T., Lu, Y.F., and Hwang, S.P.
- Source
- Full text @ PLoS One
|
The phenotype of the s1pr2as10 mutant. (A-H) Lateral views of wild-type (WT) (A, C, E and G) and s1pr2as10 mutants (B, D, F and H). Pericardial edema (B′), small eyes, and tail blisters (B′) were observed in 72-hpf s1pr2as10 mutant embryos (B). Two separate hearts (white arrows, D) were detected at 24 hpf, whereas a fused heart (white arrow, F) was observed at 48 hpf in s1pr2as10 mutant embryos. Paraffin sectioning with haematoxylin and eosin staining revealed the presence of two atria and one ventricle in s1pr2as10 mutant embryos at 72 hpf (H). (I-M) Ventral views of wild-type (WT) (I), s1pr2as10 (J), mil (K), and the intercross mutant progeny of s1pr2as10 and mil mutants (L and M) at 24 hpf are shown. The arrows indicate the positions of the hearts. (N) Cardiomyocytes were labeled via cmlc2 staining. Two representative s1pr2as10 mutant embryos are shown. Both contacting and separated cardiomyocytes were detected in the s1pr2as10 mutant embryos from the 22 ss to 28 hpf. (O) Percentages of WT and s1pr2as10 mutants containing contacting myocardia at different developmental stages. Scale bar = 100 μm. The error bars indicate the standard error. EXPRESSION / LABELING:
PHENOTYPE:
|
|
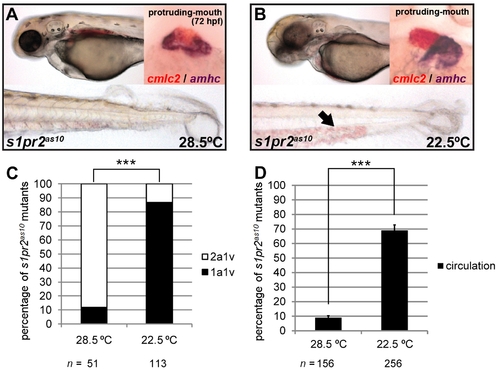
Development at low temperature rescues the s1pr2as10 cardiac phenotype. (A) s1pr2as10 mutants raised at 28.5°C contained hearts with two atria (dark purple, amhc staining) and one ventricle (red, cmlc2 staining), and presented with tail blisters at 72 hpf. (B) s1pr2as10 mutant embryos raised at 22.5°C contained hearts with a single atrium and ventricle, and exhibited blood circulation at 72 hpf (arrow). (C) Percentages of s1pr2as10 mutant embryos raised at either 28.5 or 22.5°C containing two atria and one ventricle (2a1v) or one atrium and one ventricle (1a1v) at 72 hpf. (D) Percentages of s1pr2as10 mutant embryos raised at either 28.5 or 22.5°C displaying blood circulation at 72 hpf. The error bars indicate the standard error. Statistical significance was determined using Student’s t-test. *** indicates p<0.001. PHENOTYPE:
|
|
Low temperature shifts the timing of formation of the cardiac cone. (A) Lateral views of 22-ss wild-type (WT) and s1pr2as10 mutant embryos raised at 28.5 or 22.5°C. (B) Time-lapse analyses of the medial migration of bilateral cardiomyocytes during the ~17–30-ss in Tg(cmlc2:EGFP, cmlc2:H2AFZmCherry)cy13 transgenic fish (WT) or s1pr2as10 mutant embryos raised at 28.5 or 22.5°C. Five WT or s1pr2as10 mutant embryos were analyzed for each stage. The images outlined in red indicate the stage at which cardiac cone formation took place under each condition. (C) In situ hybridization of 19-ss wild-type (WT) or 26-ss s1pr2as10 mutant embryos raised at 28.5°C or 22.5°C with a cmlc2 RNA probe. The number of embryos displaying cmlc2 staining/the total number of embryos analyzed is shown for each panel. (D) Different degrees of myocardial migration defects were observed in gata5 and bon morphants at 24 hpf. Class I (single heart tube), Class II (cardiomyocytes in close proximity but not in contact), and Class III (two separate hearts) defects are shown. (E) Percentages of each class of myocardial migration defects in 10 ng gata5-MO or bon-MO-injected embryos raised at 28.5 or 22.5°C. Scale bar = 100 μm. Statistical significance was determined using Student’s t-test. * indicates p<0.05. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of fibronectin 1 is affected by temperature. (A-H) In situ hybridization against fibronectin 1 (fn1) in 22-ss wild-type (WT) (A, B, E, F) and s1pr2as10 (as10) mutant (C, D, G, H) embryos raised at 28.5 or 22.5°C. Expression of fn1 mRNA increased at the midline region (white arrows in B and D) and bilateral LPM (black arrows in B and D) of both WT and s1pr2as10 mutant embryos raised at 22.5°C, as compared to those raised at 28.5°C (A and C). Expression of fn1 at the tail bud or yolk regions was not affected (E-H). (I) qRT-PCR revealed a trend towards increased expression of fn1 mRNA in s1pr2as10 mutant embryos raised at 22.5°C. The error bars indicate the standard error. (J) Knockdown of fn1 expression in s1pr2as10 mutants increased the percentage of 26-ss embryos raised at 22.5°C with the Class III cardia bifida phenotype. (K) Injection of human fibronectin protein into s1pr2as10 mutant embryos raised at 28.5°C partially rescued the cardia bifida phenotypes at 24 hpf. (K′) The red dye indicates the protein injection region at 14–16 hpf. Scale bars = 100 μm. Statistical significance was determined using Student’s t-test. * indicates p<0.05, *** indicates p<0.001. EXPRESSION / LABELING:
|
|
Expression of two tenascin genes is affected by temperature. In situ hybridization against tenascin-c (tnc) (A-H) and tenascin-w (tnw) (L-O) in 22-ss wild-type (WT) and s1pr2as10 (as10) mutant embryos raised at 28.5°C or 22.5°C. Expression of tnc increased in the pharyngeal arches (white arrows in B and D) and the cells between the brain and eyes of embryos raised at 22.5°C, but was unaffected in the trunk somite region (E-H). (I) qRT-PCR revealed a trend towards increased expression of tnc mRNA in s1pr2as10 mutant embryos raised at 22.5°C. (J) Knockdown of tnc expression with tnc-MO1 in s1pr2as10 mutants increased the percentage of 26-ss embryos raised at 22.5°C with the Class III cardia bifida phenotype. (K) Injection of s1pr2as10 mutant embryos raised at 28.5°C with human TNC protein partially rescued cardia bifida phenotypes at 24 hpf. (L-O) Decreased tnw expression in all tissues, including scattered epidermal cells in the head (black arrows in M and O) was observed in both 22-ss WT and s1pr2as10 mutants raised at 22.5°C. (P) qRT-PCR was used to confirm that expression of tnw mRNA is significantly reduced in 22-ss WT and s1pr2as10 mutant embryos raised at 22.5°C. (Q) Knockdown of tnw with tnw-MO1 in s1pr2as10 mutant embryos raised at 28.5°C partially rescued cardia bifida phenotypes at 24 hpf. (R) Injection of s1pr2as10 mutant embryos raised at 22.5°C with 100 pg tnw mRNA increased the percentage of 26-ss embryos with the Class III cardia bifida phenotype. Scale bars = 100 μm. The error bars indicate the standard error. Statistical significance was determined using Student’s t-test. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. EXPRESSION / LABELING:
|
|
Genetic mapping of the s1pr2as10 mutant gene. (A) Meiotic map of the s1pr2as10 locus. The numbers above the line indicate the number of meiotic recombination events out of the total number of meioses. (B) An enlarged view of the mil/edg5 locus shows the insertion in intron 1. Three primers (F1, R1, and I1) were used to confirm the insertion. (C) A 182-bp DNA fragment amplified using primers F1 and R1 was detected in wild-type (WT) and s1pr2as10 heterozygous mutants, whereas a 426-bp DNA fragment amplified using primers I1 and R1 was detected in s1pr2as10 heterozygous and homozygous mutant embryos. (D) Expression of mil/edg5 mRNA was reduced in s1pr2as10 mutants prior to 96 hpf, as revealed by qRT-PCR. WT (+, W); s1pr2as10 mutant (, M). The error bars indicate the standard error. |
|
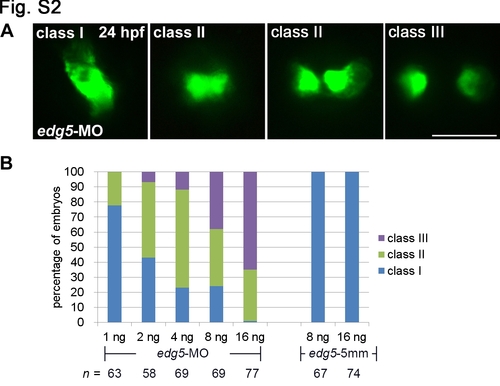
Dosage curve of an edg5-morpholino antisense oligomer (MO) and its association with cardia bifida. (A) Tg(cmlc2:EGFP, cmlc2:H2AFZmCherry)cy13 embryos injected with different doses of edg5-MO and edg5-5 mm exhibited varying degrees of myocardial migration defects, ranging from Class I (a single heart tube) to Class II (cardiomyocytes either in close proximity or in contact) and Class III (separated cardiomyocytes) at 24 hpf. Scale bar = 100 μm. (B) Percentages of Class I, II and III myocardial migration defects in Tg(cmlc2:EGFP, cmlc2:H2AFZmCherry)cy13 embryos injected with 1–16 ng of edg5-MO at 24 hpf. |
|
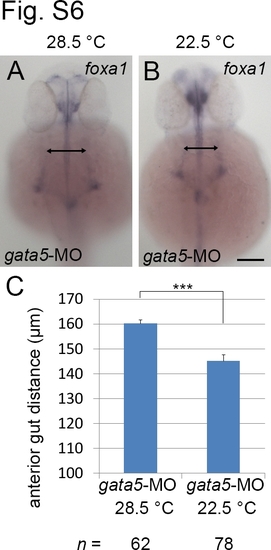
Anterior gut migration defects in gata5 morphants can be rescued by low temperature treatment. Embryos injected with 10 ng gata5 MO were incubated at 28.5°C (A) or 22.5°C (B). Embryos were harvested at prim-25 and stained with foxa1 RNA probe. (C). The distance between two lateral anterior gut tubes was significantly different between morphants incubated at 28.5°C or 22.5°C. Scale bars = 100 μm. The error bars indicate the standard error. Statistical significance was determined using Student’s t-test. *** indicates p <0.001. |
|
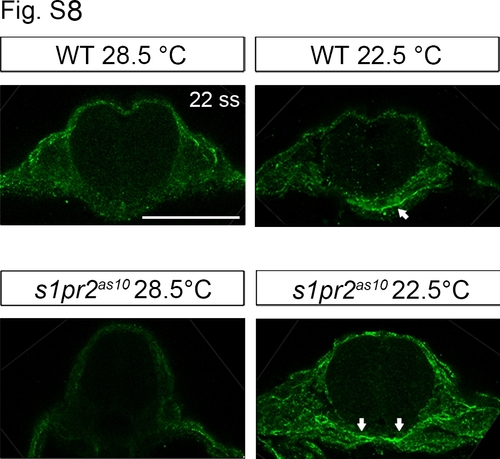
Low temperature increases fibronectin 1 expression in the midline region. Immunohistochemistry was used to demonstrate increased fibronectin 1 expression at the midline region (white arrows) of 22-ss wild type (WT) and s1pr2as10 mutant embryos raised at 22.5°C. Scale bars = 100 μm. |
|
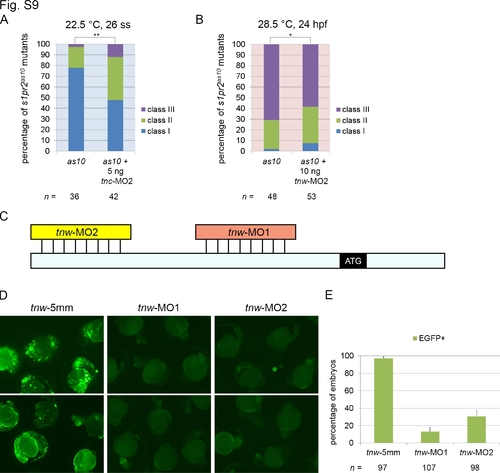
Evaluation of the roles of tnc and tnw in the mitigation of cardia bifida, and of the efficiency and specificity of tnw MOs. (A) Knockdown of tnc with tnc-MO2 in s1pr2as10 mutants increased the percentages of 26-ss embryos raised at 22.5°C with the Class II and Class III cardia bifida phenotype. (B) Knockdown of tnw with tnw-MO2 in s1pr2as10 mutant embryos raised at 28.5°C partially rescued cardia bifida phenotypes at 24 hpf. Class I (a single heart tube) to Class II (cardiomyocytes either in close proximity or in contact) and Class III (separated cardiomyocytes). Statistical significance was determined using Student’s t-test. * indicates p <0.05, ** indicates p <0.01. (C) Diagram indicating the relative binding positions of two tnw-MOs in the 5′untranslated region of tnw mRNA. (D) Green fluorescence can be detected in embryos co-injected with CMV-tnwUTR-EGFP and tnw-5mm at 24 hpf. Green fluorescence was not observed in the majority of embryos co-injected with CMV-tnwUTR-EGFP and tnw-MO1, while green fluorescence was detected in 30% of embryos co-injected with CMV-tnwUTR-EGFP and tnw-MO2. (E) Percentage of embryos expressing EGFP following co-injection of CMV-tnwUTR-EGFP with tnw-5mmMO, tnw-MO1 or tnw-MO2. The error bars indicate the standard error. |
|
Knockdown of fn1 or tnc and overexpression of tnw results in cardia bifida in wild type embryos. Wild type embryos at the one-cell zygote stage were injected with 5 ng tnc-MO1 (B), 2.5 ng fn1-MO (C), 100 pg tnw mRNA (D), or a mixture of tnc-MO1, fn1-MO, and tnw mRNA (E), and incubated at 28.5°C. Un-injected embryos were used as a control (A). Embryos were harvested at the 22 ss and stained with cmlc2. |