- Title
-
Inflammation drives wound hyperpigmentation by recruiting pigment cells to sites of tissue damage
- Authors
- Lévesque, M., Feng, Y., Jones, R., and Martin, P.
- Source
- Full text @ Dis. Model. Mech.
|
Large wounds induce hyperpigmentation in both zebrafish larvae and adults. (A) Schematics showing the wounding procedures used in this study. In zebrafish larvae, a small wound was made using a tungsten needle and then a bead was implanted through this wound into the muscle beneath the skin. In adults, a full thickness incisional wound through all skin layers was made on the fish flank using a scalpel. (B) A small needle wound to larval flank does not lead to melanocyte recruitment (Bi), whereas a wound resulting from the implantation of a bead (white asterisk) recruited melanocytes by 24 hpi (Bii). A large larval fin wound generally draws melanocytes to the site of tissue damage by 9 hours after wounding (Biii; red dashed line highlights the wound site). (C-F) Snapshot images showing time-course of progressive wound hyperpigmentation around an implanted bead in a zebrafish larva. (G-I) Still images, taken from supplementary material Movie 1, indicating the dynamic behaviour of melanocytes around the bead at 24 hpi after engraftment in a zebrafish larva. The same position is indicated by a white asterisk in sequential images to aid visualisation of a single melanocyte as it envelops the bead. (J-L) Time-course showing adult wound hyperpigmentation over a duration of 34 days post-wounding (DPW). Insets indicate how hyperpigmentation spreads into adult non-stripe domains. Scale bars: 50 μm (B); 100 μm (C-F); 25 μm (G-I); 500 μm (J-L); and 250 μm (J-L insets). |
|
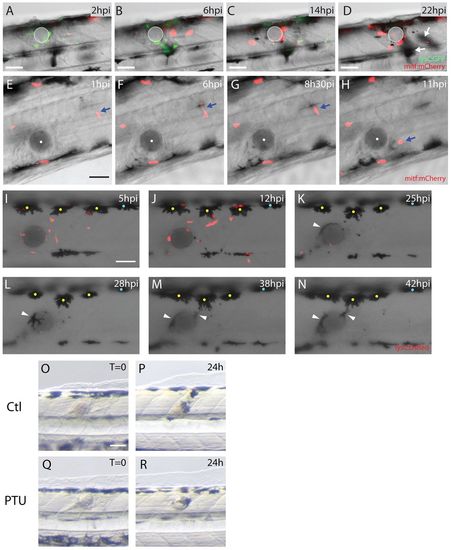
Both melanoblasts and melanocytes are recruited to the wound. (A-D) Stills taken from supplementary material Movie 2 showing neutrophils and melanoblasts as they migrate to a bead implanted in a lysC:GFP/mitf:gal4-UAS:mCherryfish larva. (A) Neutrophils (lysCGFP, green) are rapidly recruited to the wound from 2 hours after implantation. (B) By 6 hours after wounding, melanoblasts (mitf:Gal4-UAS:mCherry, red) arrive close to the bead. (C) This continues even as the inflammatory response is resolving at 14 hpi. (D) By 22 hours, melanocytes have also migrated to the wound (out of focus, indicated by white arrows). (E-H) Stills taken from supplementary material Movie 3 showing activation and migration of an individual melanoblast (mitf:mCherry, red and highlighted by a blue arrow) towards the bead. (I-N) Stills taken from supplementary material Movie 4 showing the migration of both neutrophils (red) and melanocytes (black) to the wound (bead has been implanted in a lysC:DsRed fish larva). (I) Number of neutrophils (lysC:DsRed, red) peak at the wound several hours prior to any observable response by melanocytes (yellow and blue dots). (J) First indications of melanocyte response to the wound commence at about 12 hpi (only those with yellow dots), before the final resolution of neutrophils. (K) Several melanocytes make slow progress towards the bead and by 25 hpi a smaller melanocyte has also arrived and is in close contact with the bead (white arrowhead). (L-N) Movement of small and more mature dorsal melanocytes continues for many hours, with cells extending long protrusions to reach the bead. (OR) Comparison of fish treated with PTU at the time of bead implantation versus untreated controls. In both control (O,P) and PTU-treated fish (Q,R) the wound is pigmented by 24 hpi. Scale bars: 50 μm (A-R). |
|
Inflammation precedes pigment cell migration to a wound. (A-E) Confocal images gathered at different time-points to reveal recruitment of innate immune cells to the implanted bead in a zebrafish larva. (A) By 15 minutes after implantation a small number of neutrophils (mpo:GFP, green and yellow cells) and occasional macrophages (L-plastin-positive, red) are already present in the wound vicinity. The bead is highlighted in grey with the centroid as a white dot. (B) The peak of neutrophil recruitment is at 3 hpi. (C) By 6 hpi, many innate immune cells are observed around the bead and this time is approximately the peak of macrophage recruitment. (D) Inflammation persists for some time and a large number of leukocytes can still be found close to the bead at 12 hpi. (E) Inflammation has partially resolved at 24 hpi, but a few leukocytes are still lingering around the bead. (F) Timeline highlighting the sequential recruitment of different cell lineages to the bead implant in zebrafish larvae. Full coloured lines represent the period during which cells are recruited to the bead. Dotted lines represent the period when cells are migrating away from the bead. The transition between the full and dotted line marks approximately the peak of recruitment for each cell lineage. Scale bar: 50 μm. |
|
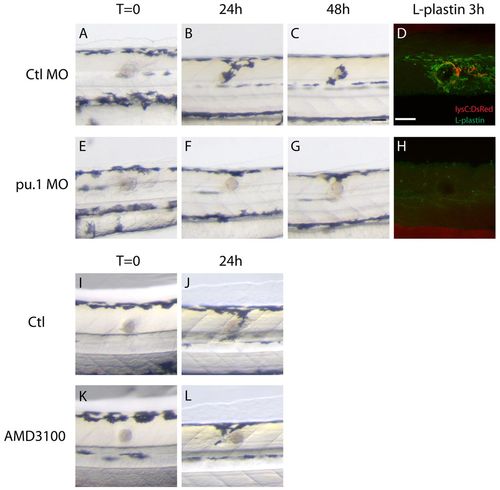
Wound recruitment of melanocytes requires innate immune cells. (A-H) Time-course of melanocyte recruitment to the bead in control and pu.1 MO-injected fish. (A-C) As shown in Fig. 1, there is a time-dependent migration of melanocytes to the wound over the first 48 hours following bead implantation in control fish. (E-G) Depletion of innate immune cells in pu.1 MO-injected fish delays and reduces the recruitment of melanocytes to the wound. (D) Immunostaining of innate immune cells with anti-L-plastin antibody shows the presence of leukocytes around the wound in control fish; neutrophils (lysC:DsRed) are red and macrophages (L-plastin-positive cells) are green. (H) No leukocytes are evident in pu.1 MO-injected fish. (I-L) Compared with control fish (I,J), AMD3100-treated fish do not show a reduction in wound hyperpigmentation at 24 hpi (K,L). Scale bar: 50 μm (A-L). |