- Title
-
A Model for Cleavage Plane Determination in Early Amphibian and Fish Embryos
- Authors
- Wühr M., Tan E.S., Parker S.K., Detrich H.W. 3rd, and Mitchison T.J.
- Source
- Full text @ Curr. Biol.
|
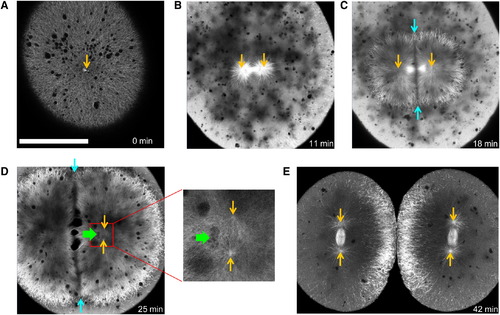
EMTB-3GFP Transgenic Zebrafish Embryos Allow Live Imaging of Microtubule Organization in Large Cells Orange arrows indicate positions of centrosomes.(A) Shortly after fertilization, sperm aster expands throughout the cell. The scale bar represents 200 μm. (B) Before metaphase, sperm aster breaks down and first mitotic spindle forms. (C) During anaphase-telophase, astral microtubules grow out, and centrosomes move apart. An interaction zone forms in the plane where sister asters contact each other (between blue arrows). (D) Centrosomes separate and align in the direction of the future spindle during late telophase (see enlargement). The centrosomes in the left aster are out of focus. Nuclei (green arrow) follow centrosomes, lagging behind. (E) Second mitotic spindles assemble after cytokinesis (E is taken from different embryo). See also Figure S2 and Movies S1 and S2. |
|
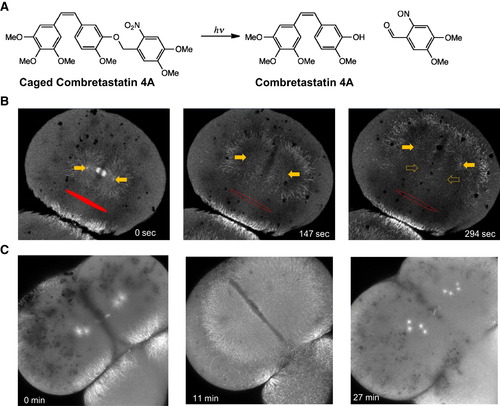
Aster Movement Depends on Dynein-Dependent Pulling Forces (A) Structure of caged combretastatin 4A. (B) EMTB-3GFP zebrafish embryos were incubated in caged combretastatin and subjected to UV irradiation in defined region (marked in red). Within seconds, microtubules depolymerized selectively on the irradiated side. The remaining aster moved away from the irradiated region, arguing for pulling forces on asters. Full arrows mark the positions of centrosomes; hollow arrows on right mark their original positions. (C) Injection of p150-CC1 blocks aster movement. Asters still grew out and broke down under cell-cycle control but lost their ability to move or orient centrosomes. See also Figure S3 and Movies S3 and S4. |
|
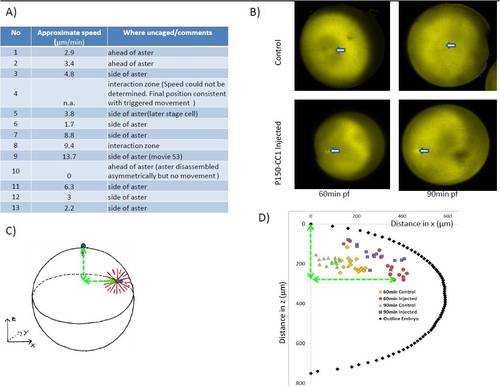
Related to Figure 3. A) List of experiments in which microtubules were depolymerised locally in zebrafish embryos, together with the speed of aster movement. Speed was measured (approximately) by tracking the movement of fixed parts at the center of the aster (the centrosome or mid-body). Positive speeds indicate movement away from the irradiated zone. Movement towards the region of uncaging (which we did not observe) would have been reported as negative speed. In 12/13 experiments the aster moved away from the irradiated zone, in 1/13 it did not move and in no case was movement towards the zone observed. B-D) Sperm-aster in frog embryos depends on dynactin to move centrosome: Embryos were synchronously fertilized and fixed 60 and 90 min after fertilization. B) By staining against tubulin sperm aster formation and centrosome movement could be followed in p150-CC1 injected and control embryos. Arrow indicated position of centrosome. C) Because centrosome movement is three-dimensional, centrosome position was recorded relative to the animal pole at the top of the embryo. D) Centrosome position of injected and control embryos were plotted with circumference of one embryo. In control embryos centrosomes move from the periphery towards the cell’s center while p150-CC1 injected embryos centrosomes were located close to the cortex |