- Title
-
The RhoA GEF Syx is a target of Rnd3 and regulated via a Raf1-like ubiquitin-related domain
- Authors
- Goh, L.L., and Manser, E.
- Source
- Full text @ PLoS One
|
Rnd3 associates with Syx in mouse embryonic stem cells. (A) Expression analysis of Rnds evaluated by RT-PCR from mES cells, mouse embryonic fibroblast (mEF) cells, embryoid bodies (EB) or plated differentiated mES cells (PD). (B) Identification of Rnd3-associated proteins. Rnd3 TAP-tagged proteins from transiently transfected mES cells using CBP-SBP vector (see methods). The purified proteins by coomassie staining is shown with identification by LC-MS analysis. (C) Expression levels of Rnd3 and Syx in NIE-115, HeLa and mES cells. Cell lysates were resolved by SDS-PAGE and immunoblotted with anti-Rnd3 and anti-Syx. (D) Endogenous localizations of Rnd3 and Syx in HeLa cells immuno-labeled with anti-Syx and Rnd3 antibodies. Rnd3 and Syx are both localized near the perinuclear region. Bar = 10μM. |
|
Analysis of the Rnd3-Syx interaction. (A) Schematic representation of Flag-SBP tagged Rnd3. The motifs of Rho family proteins are indicated: P-loop, the switch I and II (SI and SII), and Rho insert (RI) region. Flag (FL) and streptavidin binding peptide (SBP) tags are indicated. Sequence alignment of Rnd3 proteins showed that the N-terminal of zebrafish Rnd3a resembles more closely to mouse Rnd3. Identical residues are shaded in grey. Blue arrowhead denotes G14 in RhoA. Residue effector region is underlined. The accession numbers of mouse Rnd3 (NP083086), zebrafish Rnd3a (BC059452), Rnd3b (BC076009) and mouse RhoA (NP058082) are in brackets. (B and C) Characterization of Syx binding to Rnd isoforms. 293T cells were co-transfected with expression vectors encoding SBP-Flag Rnd1, Rnd2 or Rnd3 and full-length Syx or Syx(1–800) as indicated. Lysates were subjected to SBP pulldown and immunoblotting. (D) Rnd3 does not interact with MyoGEF. 293T cells were co-transfected as for (B) with Flag-MyoGEF and assessed for interaction. (E) Selective association of Syx with Rnd3. Co-expression of SBP-Flag RhoA(GV14), Cdc42(G12V), Rac1(G12V) or Rnd3 with Syx(1–800) and immunoblotted for Flag epitope. |
|
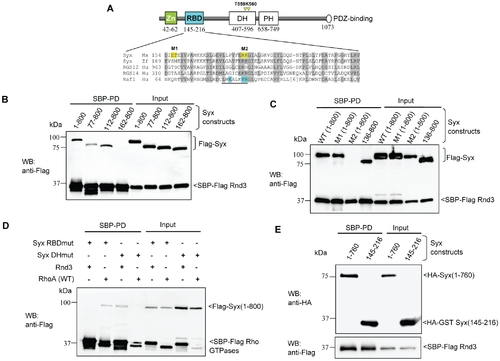
Characterization of the interaction between Rnd3 and Syx. (A) Schematic of domains identified in Syx: including zinc-finger, Rnd3-binding (in cyan), Dbl homology (DH), pleckstrin homology (PH) and PDZ-binding motif. Sequence alignment of Raf1-like RBDs in mouse Syx (AAU04953), zebrafish Syx (XM_686228.1), human RGS12 (NP_002917), RGS14 (NP_006471) and Raf1 (AAA60247). Conserved residues are shaded in grey; a key helix is underlined; amino acid side chains contacting Ras are in cyan and residues mutated in this study are in yellow. The catalytic residues T559/K560 in Syx DH domain are denoted by yellow arrowheads. (B) Rnd3 interacts with the N-terminal of Syx. 293T cells were co-transfected with SBP-Flag Rnd3 and indicated Flag-Syx constructs. The purified protein complex was immunoblotted to visualize bound Syx. (C) R178 and K179 of Syx are critical Rnd3 binding residues. 293T cells were co-transfected as for (B) with the indicated constructs and assessed for interaction. M1 and M2 refer to the mutant constructs, E156AT157A and R178EK179D. (D) Rnd3 and RhoA (WT) interact with Syx via the RBD and DH domain, respectively. The mutant constructs, R178EK179D and T559EK560E are represented by Syx RBDmut and Syx DHmut, respectively. (E) Rnd3 interacts with Syx(145–216). |
|
Zebrafish Syx is closely related to mammalian Syx1b and expressed from epibolic stages. (A) Zebrafish Syx interacts with mouse Rnd3 via its RBD domain (residues 173–246). (B) Expression of Syx and Rnds mRNA. RT-PCR analysis showing mRNA transcript profiles of zebrafish Syx, Rnd3a and Rnd3b at different developmental stages indicated. Primers to Syx (nucleotide 2640–3120) (XM001923778), Rnd3a (nucleotide 1–732) (BC059452) and Rnd3b (nucleotide 1–672) (BC076009) were used. (C) Schematic representation of zebrafish Syx, including the conserved RBD, DH/PH domains and PDZ-binding motif. The N-terminal region of zebrafish Syx resembles more closely to mammalian Syx1b than the published Syx1a. Conserved residues are in light grey and identical residues in dark grey. The zinc finger motif is underlined and the conserved cysteine residues marked (*). Database numbers for zebrafish Syx (XM_686228.1), mouse Syx1b (B1AS67), human Syx1b (094827), mouse Syx1a (AAU04953) and human Syx1a (AK299523) are in brackets. (D) RT-PCR revelas that Syx 1b is more highly expressed in mouse NIE115, ES and human HeLa cells. RT-PCR from alternative start sites to a common downstream primer (nucleotide 495). |
|
Loss of Syx causes gastrulation defects and death that can be rescued by mouse Syx1b mRNA. (A) Typical phenotypes observed in zebrafish embryos at 16 and 25 hpf. At 16 hpf, Syx depleted embryos displayed shortened AP axis, indicative of gastrulation defects. Co-injection of Syx MO with mouse Syx1b mRNA reduced both death and gastrulation defects. Co-injection of Syx E164A/R165D mRNA was more effective than wildtype (WT) Syx. At 25 hpf, Syx morphants had shorter body length and kinky tail (though most died). Rescued phenotypes of WT and E164A/R165D at 25 hpf resembled un-injected controls. (B and C) Quantification of embryos (n is the total number scored) showing penetrance of Syx morphants and rescue through co-injection of Syx1b WT or E164A/R165D RNA at 16 and 25 hpf. PHENOTYPE:
|
|
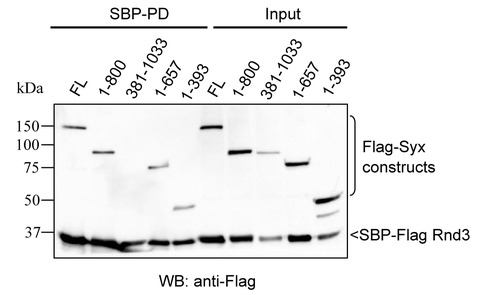
Rnd3 interacts with the N-terminal of Syx. 293T cells were co-transfected with SBP-Flag Rnd3 along with the indicated Flag-tagged Syx constructs. Cells were lysed 24 h post-transfection, and cleared lysates were subjected to SBP pulldown. The purified protein complex was analyzed by immunoblotting with anti-Flag antibody to visualize the associated proteins. |
|
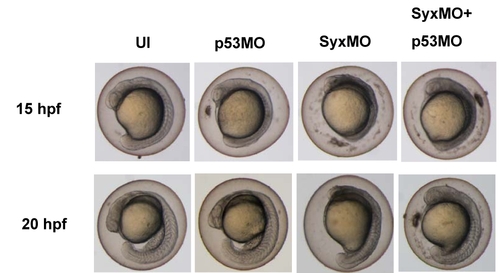
Syx morphant phenotype was maintained in the presence of p53 MO. Typical phenotypes observed in zebrafish embryos at 15 and 20 hpf. At 15 hpf, Syx depleted embryos displayed shortened AP axis. Co-injection of Syx MO (2.5 ng) with p53 MO (4.5 ng) did not reduce death and gastrulation defects. At 20 hpf, Syx morphants and those coinjected with p53 have shorter body length (though most died). Phenotypes of p53 MO injected embryos resembled un-injected controls. PHENOTYPE:
|