- Title
-
Regulation of membrane progestin receptors in the zebrafish ovary by gonadotropin, activin, TGF-beta and BMP-15
- Authors
- Tan, Q., Zagrodny, A., Bernaudo, S., and Peng, C.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
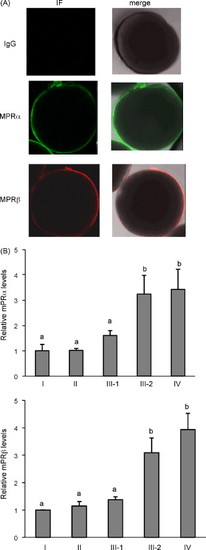
(A) Localization of zebrafish mPRα and mPRβ proteins in fully grown oocytes by immuofluorescence staining and confocal microscopy. The follicular layer was removed from zebrafish follicles and the oocytes were probed with antibodies specific for mPRα and mPRβ. Both mPRα (green) and mPRβ (red) were localized on the oocyte membrane. No immuofluorescence signal was observed using rabbit-IgG as the primary antibody. IF, immunofluorescent signals; merge, IF signal merged with a bright field picture of the oocyte. (B) Expression of mPRα and mRPβ during follicle development. Protein samples were prepared from follicles at various stages of development and subjected to Western blotting probed by mPRα, mPRβ, and α-tubulin antibodies. Data from each experiment is normalized to stage I and presented as mean ± S.E.M. (n = three experiments). Different letters denote statistical significance (p < 0.05). EXPRESSION / LABELING:
|
|
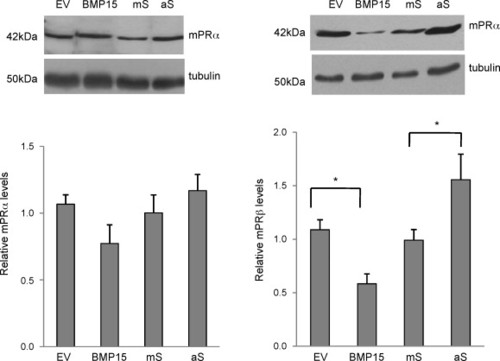
BMP-15 inhibits mPRβ, but not mPRα expression. To overexpress BMP-15, oocytes were microinjected with plasmid DNA carrying the coding sequence of zebrafish BMP-15 (1 ng/follicle) or equal amount of the empty vector as the negative control. To knockdown BMP-15, oocytes were injected with an antisense oligonucleotide (0.5 ng/follicle) targeting the translation initiation site of BMP-15. A missense oligonucleotide was used as the negative control. Proteins were extracted at 18 h after microinjection and expression of the mPRα and mPRβ was detected by Western blot analysis using mPRα and mPRβ antibodies. Equal loading was confirmed by Western blotting probed with an α-tubulin antibody. Data represent mean ± S.E.M. of five experiments.*p < 0.05. EV, empty vector control. aS, antisense oligonucleotides; mS, missense oligonucleotides. EXPRESSION / LABELING:
|
|
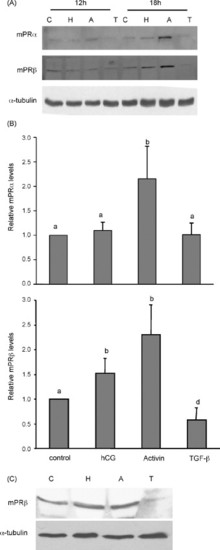
Regulation of mPRα and mRPβ by hCG (H), activin-A (A) and TGF-β1 (T). (A) Mid- to late growth phase follicles were treated with control medium (C), hCG (100 ng/ml), activin-A (100 ng/ml), TGF-β1 (10 ng/ml), for 12 and 18 h, and proteins were extracted from intact follicles and subjected to Western blotting. Equal loading was confirmed by Western blotting probed with an anti-α-tubulin antibody. (B) Histograms of normalized densitometry data for the 18 h time point (mean ± S.E.M.) from four experiments. Different letters denote statistical significance (p < 0.05). (C) Follicles were treated with hCG, activin-A, and TGF-β1 for 18 h and follicular cell layer was removed. Proteins were extracted from oocytes and Western blotting performed using mPRβ and α-tubulin antibodies. |
|
Dose-dependent effect of hCG on mPR expression. Mid- to late growth phase follicles were treated with Cortland′s medium, or hCG at different concentrations (10, 100, and 1000 ng/ml) for 18 h. Proteins were extracted and expression of mPRα and mPRβ was detected by Western blot analysis. Equal loading was confirmed by Western blotting probed with an anti-α-tubulin antibody. Histograms show normalized densitometry data (mean ± S.E.M.) from three experiments. *p < 0.05 vs. control. #p < 0.05 vs. control and hCG 10 ng/ml groups. |
|
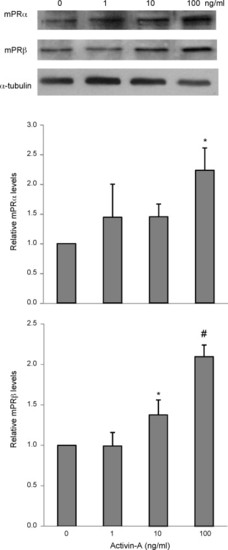
Activin-A increases mPRα and mPRβ expression in a dose-dependent manner. Mid- to late growth phase follicles were treated with Cortland′s medium or activin-A at a dose of 1, 10, or 100 ng/ml for 18 h. At the end of the experiment, proteins were extracted from follicles and expression of mPRα and mPRβ was determined by Western blotting probed by anti-mPRα and mPRβ antibodies. Equal loading was confirmed by Western blotting probed with an anti-α-tubulin antibody. Histograms show normalized densitometry data (mean ± S.E.M.) from three experiments. *p < 0.05 vs. control. #p < 0.05 vs. control and activin 1 ng/ml groups. |
|
TGF-β1 inhibits mPRβ expression in a dose-dependent manner. Mid- to late growth phase follicles were treated with Cortland′s medium, or TGF-β1 at 0.1, 1, or 10 ng/ml for 18 h. Proteins were extracted and expression of mPRα and mPRβ was detected by Western blot analysis using anti-mPRα and mPRβ antibodies. Equal loading was confirmed by Western blotting probed with an anti-α-tubulin antibody. Histograms show normalized densitometry data (mean ± S.E.M.) from three experiments. *p < 0.05 vs. control. |
Reprinted from Molecular and Cellular Endocrinology, 312(1-2), Tan, Q., Zagrodny, A., Bernaudo, S., and Peng, C., Regulation of membrane progestin receptors in the zebrafish ovary by gonadotropin, activin, TGF-beta and BMP-15, 72-79, Copyright (2009) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.