- Title
-
Promoting notochord fate and repressing muscle development in zebrafish axial mesoderm
- Authors
- Amacher, S.L. and Kimmel, C.B.
- Source
- Full text @ Development
|
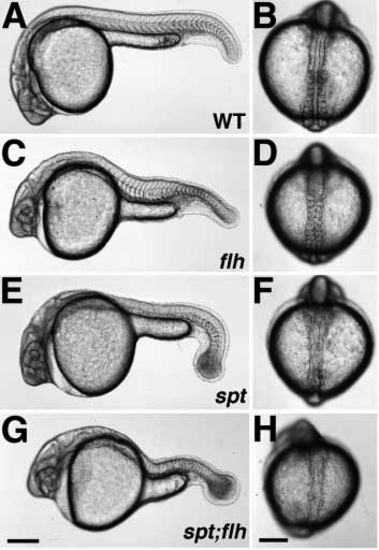
Phenotypes of live embryos during pharyngula (24 h) (A,C,E,G) and segmentation (13-14 h) (B,D,F,H) stages. In wild-type embryos (A,B), the notochord is flanked on either side by the developing somites, which give rise largely to myotomal muscle. In flh- embryos (C,D), the notochord is missing and the somites (and later, muscle) are fused across the midline. Like spt- embryos (E,F), spt-;flh- embryos (G,H) lack trunk somites, but have tail somites. Although a notochord is present in both spt (E,F) and spt;flh (G,H) mutant embryos, it is incomplete in the double mutants. The embryo shown in H is an example of moderate notochord rescue; almost 90% (30/34) of spt-;flh- embryos have a detectable notochord primordium during early to midsegmentation stages. In about one-third (11/34) of spt-;flh- embryos, notochord rescue is so extensive that double mutant embryos are indistinguishable from spt single mutant embryos, at least at the level of the dissecting microscope. (A,C,E,G and B,D,F,H) Lateral and dorsal views, respectively. Scale bars, 250 um (left panels), 200 um (right panels). PHENOTYPE:
|
|
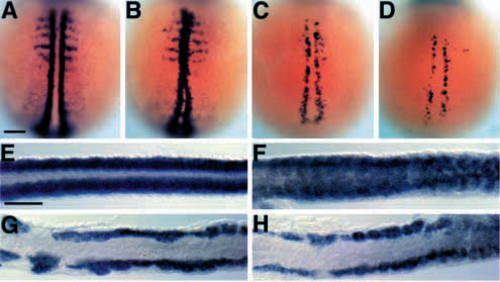
myoD is not expressed in the spt-;flh- midline. (A-D) Dorsal views of myoD expression at early segmentation stages (2-4 somites, 10.7-11.3 h, anterior to the top). In wild-type embryos (A), myoD is expressed in a two bilateral rows of adaxial cells and more laterally in each somite (Weinberg et al., 1996); in flh- embryos (B), myoD is expressed in the midline (Halpern et al., 1995). There are fewer myoD-expressing cells in spt- (C; Weinberg et al., 1996) and spt-;flh- (D) embryos, but they are confined to mesoderm flanking the developing notochord. (E-H) Dorsal views of myoD expression at late segmentation stages (26 somites, 22 h, anterior to the left). In wild-type embryos (E; Weinberg et al, 1996), myoD is expressed in bilateral rows of muscle that flank the notochord. In flh- embryos (F), myoD is expressed in midline mesoderm. myoD expression in spt- (G) and spt-;flh- (H) embryos is strikingly similar, although occasionally a few myoD-positive midline cells are observed in the double mutant tail. Scale bars, 100 um. |
|
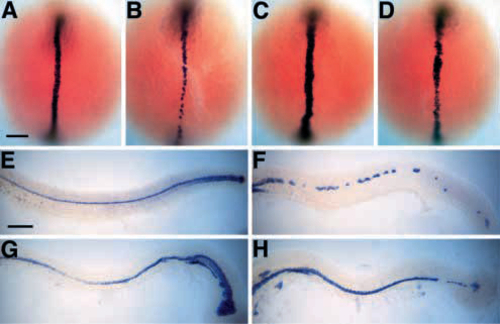
The spt-;flh- floor plate is nearly continuous. (A-D) Dorsal views of shh expression during early segmentation (6-8 somites, 12- 13 h, anterior to the top). shh is expressed in the developing notochord and floor plate of wild-type (A; Krauss et al., 1993) and spt- (C) embryos. In flh- embryos (B), shh expression is seen only in presumptive floor plate cells; however, in spt-;flh- embryos (D), shh is expressed in both in floor plate and in anterior midline mesodermal cells (presumptive notochord). The extent of shh expression in spt-;flh- midline mesoderm is variable; most spt-;flh- embryos look as shown (13/21, 62%); however, some spt-;flh- embryos have less extensive shh expression (3/21, 14%), whereas other spt-;flh- embryos with more extensive staining were recovered from the spt- class (5/21, 24%). Slightly fewer spt-;flh- embryos were observed than expected (21/422 versus 26/422), raising the possibility that shh expression in few spt-;flh- embryos is so extensive that they are indistinguishable from spt- embryos. (E-H) Lateral views of shh expression at the completion of the segmentation period (25 h, anterior to the left). In wild-type (E; Krauss et al., 1993) and spt- (G) embryos, shh is expressed in the floor plate and posterior notochord. In spt- embryos, shh-expressing cells are also found ventral to the notochord. shh expression in spt-;flh- embryos (H) reveals a nearly continuous floor plate, which is in great contrast to the patchy shh expression observed in the floor plate ‘islands’ of flh- embryos (F; Halpern et al., 1995, 1997; Talbot et al., 1995; Odenthal et al., 1996; Beattie et al., 1997). The spt-;flh- embryo shown in H is a typical example (13/24, 54%); other spt-;flh- embryos have either more extensive (6/24, 25%) or less extensive (5/24, 21%) shh expression in the tail. Scale bars, 100 um. |
|
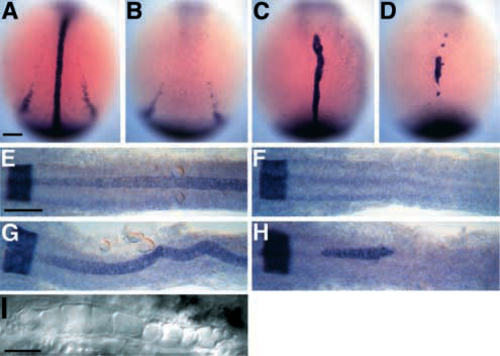
Anterior spt-;flh- midline cells express the essential notochord gene, ntl. (A-D) Dorsal views of ntl expression during early segmentation stages (4-6 somites, 11.3-12 h, anterior to the top). ntl is expressed in wild-type embryos (A) in the developing notochord and in the tailbud (Schulte-Merker et al., 1992, 1994) and, in these overstained embryos, in the more laterally located presumptive pronephric ducts. In flh- embryos (B), midline ntl staining is conspicuously missing. In about half the flh- embryos, there is anterior midline ntl expression in approximately 1-6 cells (not shown). ntl expression in spt- embryos (C) is approximately normal (Hammerschmidt et al., 1996a), except the presumptive pronephros domain is reduced or absent. Midline ntl expression is observed in all spt-;flh- embryos (D), typically in one large anterior patch, and occasionally in a few additional smaller patches, like those shown here. Many spt-;flh- embryos (10/20, 50%) look as shown; however, a significant number (8/20, 40%) have more extensive ntl expression, some of which were recovered from the spt- class after staining. A few spt-;flh- embryos have only about 10 ntlpositive cells (2/20, 10%). The observed number of spt-;flh- embryos was as expected (20/316). (E-H) Dorsal views of krox-20 and ntl staining during later segmentation stages (22 somites, 20 h, anterior to the left) when notochord differentiation is well underway. krox-20 is expressed in a broad band at the extreme left of each photograph that marks a hindbrain segment, rhombomere 5, in all embryos (Oxtoby and Jowett, 1993). In wild-type embryos (E), ntl is expressed in the entire notochord, beginning at the level of rhombomere 4 or 5 and extending posteriorly. Expression is beginning to fade in the anteriormost notochord. No midline ntl expression is observed in any flh- embryos (F). In spt- embryos (G), ntl expression reveals a wide, kinked notochord. Patches of anterior ntl expression are observed in spt-;flh- embryos (H). Most spt-;flh- embryos (8/12) show expression in a patch containing about 20-60 midline cells underlying the posterior half of the hindbrain; three of these embryos have additional smaller clumps located either more anteriorly (as shown in H, see small clump just anterior to rhombomere 5) or more posteriorly at midtrunk levels. A few double mutants (2/12) have two small patches of ntl-expressing midline cells each containing 2-5 cells, and the remaining double mutants (2/12) show no ntl expression at this stage. (I) 30 h Nomarski view of spt-;flh- notochord cells. Scale bars, 100 um (A-H), 50 um (I). |
|
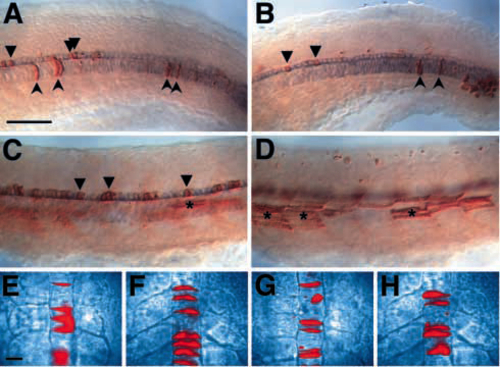
flh mutant cells can make notochord when transplanted into a wild-type environment. Blastula transplantations were performed as described in the Materials and Methods. (A-D) Lateral views of transplants at 24-28 h; shh-expressing cells are shown in blue and the transplanted cells are shown in brown. (A) A control transplant showing transplanted wild-type cells contributing to wild-type host notochord (concave arrowheads) and floor plate (arrowheads). (B) A transplant showing flh- cells contributing to wild-type host notochord and floor plate. (C) A transplant showing contribution of flh- cells to wild-type host floorplate, but not to the notochord. (D) The same embryo as in C, but a different focal plane to show the large number of flh- donor muscle cells (asterisks). (E-H) Images of wild-type (E,F) and flh- (G,H) transplanted cells (shown in red) in live wildtype hosts. Embryos were photographed several times during the segmentation period, beginning at the 3-somite stage (11 h) (E,G) and ending at the 20-somite stage (19 h) (F,H). Scale bars, 50 um (AD), 25 um (E-H). |