- Title
-
Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis
- Authors
- Kramer-Zucker, A.G., Olale, F., Haycraft, C.J., Yoder, B.K., Schier, A.F., and Drummond, I.A.
- Source
- Full text @ Development
|
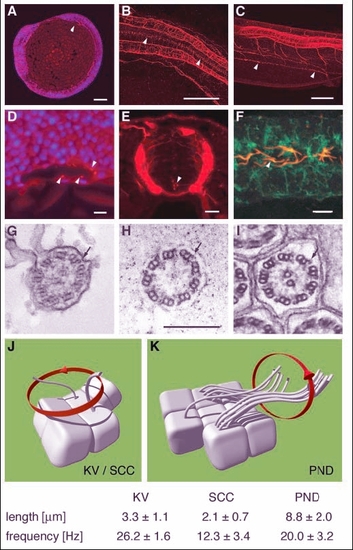
Apical cilia are present in Kupffer's vesicle, the central canal of the spinal cord and pronephric ducts. Immunostaining of acetylated tubulin. (A) Apical cilia are present in cells lining Kupffer's vesicle at the 8-somite stage (arrowhead) in midline longitudinal sections. (D) Kupffer's vesicle; higher magnification (DAPI nuclear staining in blue). (B) Ependymal cells along the central canal bear cilia at 24 hpf (arrowheads). (E) Cross section at 44 hpf; cilia arise from all cells of the spinal central canal. (C) Cilia can also be seen in the pronephric duct at 48 hpf (arrowheads). (F) Cells double stained for acetylated tubulin and the alpha1 subunit of the NaK-ATPase confirmed the apical position of the pronephric cilia. (G-I) EM cross sections of cilia in Kupffer's vesicle (G) show a 9+2 structure; ependymal cell cilia (H) are 9+0 in structure; pronephric cilia (I) are 9+2 with clear dynein outer arms (arrows). Cilia beat pattern: (J) The cilia in the of the spinal central canal and Kupffer's vesicle rotate in an counterclockwise orientation. (K) In the pronephric duct monociliated and multiciliated cells can be observed. Their cilia beat in rotation like a corkscrew with an undulating appearance along their longitudinal axis. Mean values for cilia length and beat frequency are given for comparison. Scale bars: 100 Ám in A-C; 10 Ám in D-F; 250 nm in G-I. KV, Kupffer's vesicle; PND, pronephric duct; SCC, spinal central canal. |
|
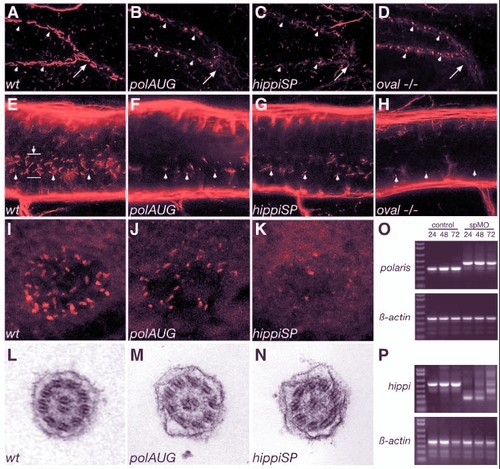
Cilia structure is altered in IFT morphant embryos. A whole-mount confocal immunostaining of acetylated tubulin in 44 hpf embryos shows apical cilia in the pronephric ducts (A-D), the spinal canal (E-H) and Kupffer's vesicle (I-K). At 44 hpf polaris and hippi morphants (B,C), as well as oval homozygous mutant embryos (D), exhibit severely shortened cilia (arrowheads) compared with wild type (A). For reference, arrows in A-D indicate the point were the pronephric ducts merge at the cloaca. (E-H) Cilia (arrowheads) in the central canal (lumen indicated by arrow in E) of the spinal cord are also shortened or absent in polaris (F) and hippi (G) morphants and in oval-/- embryos (H) compared with wild type (E). Immunostaining of pronephros/central canal: anterior is to the left, dorsal to the top. Kupffer's vesicle cilia in polaris (J) and hippi (K) morphant embryos are greatly reduced compared with control (I). (L,M) Ultrastructure of the pronephric cilia in wild-type (L), polaris (M) and hippi (N) morphants show a typical 9+2 microtubule doublet pattern. (O) Molecular analysis of the effectiveness of SP-morpholinos inducing splice defects: RT-PCR of single embryos generates a 354 bp polaris fragment in control embryos, bridging part of exon 1 to part of exon 5 at 24, 48, 72 hpf (lane M, 1 Kb Plus DNA Ladder). polarisSP-injected embryos analyzed with the same primer sets at the same timepoints show a larger amplicon of 457 bp caused by a non-splicing of intron 2, which encodes a premature stop codon; the lower wild-type band recovers over time. Lower panel: RT-PCR of ▀-actin of the same RNA samples. (P) RT-PCR of hippi mRNA results in a 553 bp fragment in control embryos, whereas the amplicon is reduced in size in the hippiSP-treated embryos, indicating an in-frame deletion of exon 2 and 3 (lower band, 260 bp) or an out-of-frame deletion of exon 2 only (middle band, 378 bp); there is recovery of the wild-type band over time. |
|
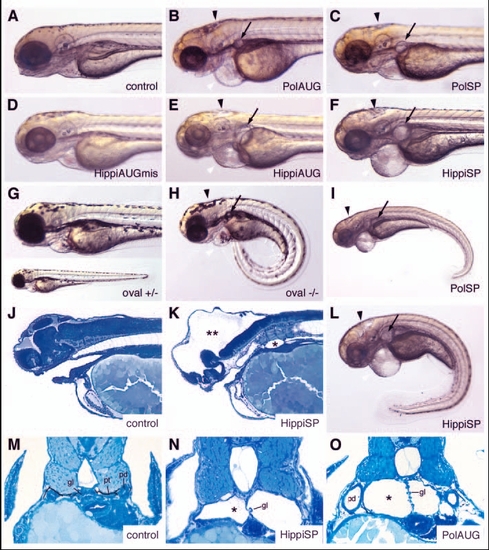
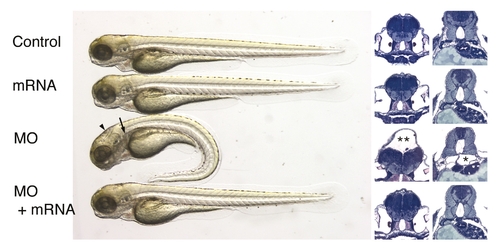
IFT morphant phenotype: kidney cysts and hydrocephalus. Disruption of polaris function by injection of polarisAUG (B) and polarisSP (C,I) results in hydrocephalus (black arrowhead), pronephric cyst formation (arrow), pericardial edema (white arrowhead), and ventrally bent body axis at 72 hpf compared with non-injected control embryos (A). This phenotype imitates the oval homozygous mutant (H), in which the polaris gene is mutated. Heterozygous embryos are indistinguishable from wild-type controls (G). Embryos injected with the control hippiAUG mismatch morpholino have a normal morphology (D), whereas hippiAUG (E) and hippiSP (F) cause a phenotype similar to the polaris morphants and the oval homozygous mutant. (J) Wild-type embryo in longitudinal section. (K) hippiSP morphant embryo showing severe hydrocephalus (**) and kidney cyst (*). (L) hippiSP morphant embryo showing axis curvature, cysts (arrow) and hydrocephalus (arrowhead). (M) Histological cross sections of a 72 hpf control embryo show the midline fused glomerulus, pronephric tubules and pronephric ducts on either side. (N,O) hippiSP morphant embryos at 72 hpf show a cystic dilatation (*) of the pronephric tubules with a stretched glomerulus in the midline. (O) polarisAUG morphants show kidney cysts (*) and distension of the pronephric ducts. gl, glomerulus; pd, pronephric ducts; pt, pronephric tubules. |
|
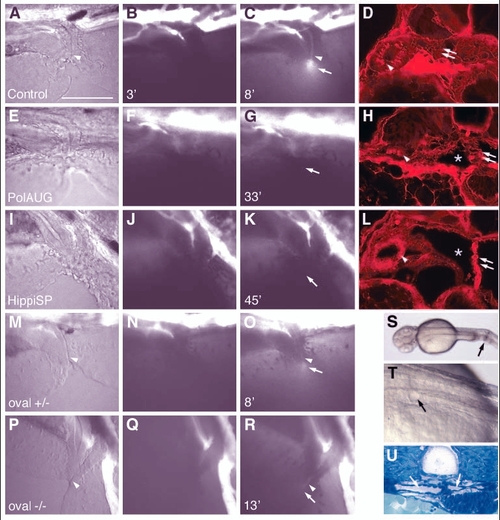
Fluid flow is impaired by lack of normal cilia movement in the pronephros. Living embryos were injected with 5% tetramethylrhodamine-conjugated 70 k MW dextran into the circulation. After passage of the pronephric kidney, the dye was excreted at the cloaca (C, arrow). The images of the first column (A,E,I,M,P) are transmitted light images. The images in the second column (B,F,J,N,Q) were taken at 2-3 minutes post-injection, while the images in the third column (C,G,K,O,R) were captured when maximum excretion was reached. The time after injection that the image was captured is indicated in the bottom left of each panel. No fluorescent dye excretion via the cloaca was observed at timepoints >30 minutes in polarisAUG morphants (n=9) or hippiSP morphants (n=9), whereas in the control embryos excretion was observed in 22 individuals (n=27). On histology of the same embryos, all showed endocytic uptake of the dye in anterior duct cells (arrowhead) (D,H,L), indicating that the dye had been filtered via the glomerulus (double arrows) into the cyst lumens (*). In oval heterozygous embryos, excretion started at 5.3▒0.4 seconds (n=2) (O), and 3 out of 5 oval homozygous embryos showed weak dye excretion at 13.7▒5.5 seconds (n=3), whereas 2 did not show a visible output. In A-R anterior is to the left and dorsal is to the top. Mechanical obstruction of the pronephric ducts close to the cloaca (S, arrow) causes cystic distension of the anterior pronephric tubules within 30 minutes (T, arrow). The dilated tubules/glomerulus can be seen in cross sections (U, arrows). Scale bar: 100 Ám. |
|
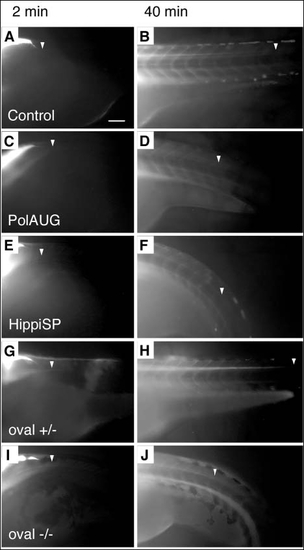
Fluid flow is impaired by lack of normal cilia movement in the central canal of the spinal cord. BDM-pretreated embryos were injected with 5% tetramethylrhodamine-conjugated 70 k MW dextran into the fourth brain ventricle and dye distribution along the central canal of the spinal cord was recorded at various timepoints; shown are 2 and 40 minutes post-injection. Control (A,B) and oval heterozygous (G,H) show a distribution of the dye up to an anterior-posterior level of the tip of the yolk extension at 40 minutes (arrowheads) (B,H), whereas polarisAUG morphant (C,D), hippiSP morphant (E,F) and oval homozygotes (I,J) show reduced dye migration. Anterior to the left. Scale bar: 100 Ám. |
|
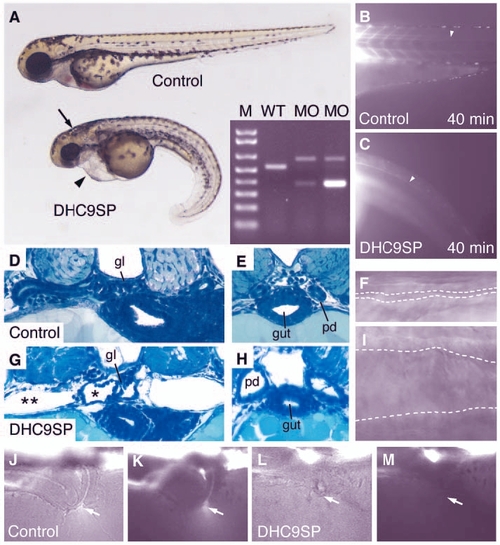
Dynein heavy chain 9 knockdown morphants show abnormal cilia movements and phenotypic changes similar to the IFT morphants. A morpholino targeting the splice-donor site of the exon coding for the P1-domain of dhc9 causes kidney cysts, hydrocephalus (arrow) and axis curvature (A). Sequencing RT-PCR of aberrant splice products (A, inset) revealed non-splicing of the adjacent intron with a premature stop codon (upper band) and an out-of-frame deletion of the P1-domain coding exon (lower band). The transport of injected fluorescent dye along the central canal of the spinal cord (B,C) is impaired in dhc9SP morphants (C) versus control (B). Histologically, Dhc9P1SP morphant embryos show distension of the tubules near the glomerulus (G) and dilated ducts (H) compared with wild-type control (D,E). The dilatation of the duct can also be seen in frames taken from Movie 7 (see supplementary material) (F, wild type; I, Dhc9P1SP morphant). (J-M) Dye excretion via the urine was not detected in dhc9SP morphants (arrows in L,M) versus control (J,K). |
|
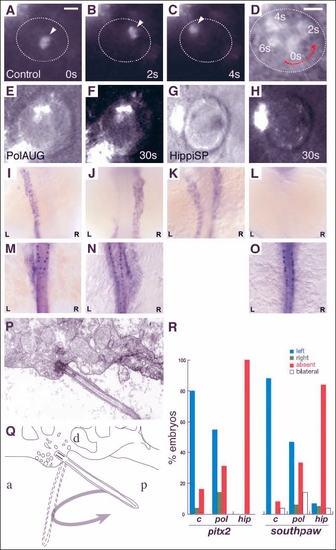
Impaired fluid flow in Kupffer's vesicle is associated with defects in laterality. Embryos at the 8-10 somite stage were dechorionated and fluorescent beads were injected into Kupffer's vesicle. Control embryos showed a rotating movement of bead aggregates in a counterclockwise orientation (arrowheads in A-C) when viewed dorsally. Relative timepoints in seconds are indicated in the bottom right of each panel. The wall of Kupffer's vesicle is indicated by dotted lines. (D) Superposition and enlargement of frames (A-C) with an additional transmitted light frame showing the counterclockwise direction of movement. None of the injected morphant embryos (polarisAUG and hippiSP) showed this phenomenon (E-H). Abnormal expression of the laterality markers pitx2 and spaw in polarisAUG and hippiSP embryos (I-O,R). In situ experiments were performed on 14-somite (spaw) and 20-somite (pitx2) embryos. Dorsal views of the lateral plate mesoderm are shown with the different expression patterns seen in polarisAUG embryos. southpaw was expressed on the left (I), right (J) or bilaterally (K), or in many cases absent (L). pitx2 shows the same patterns (M, left-sided; N, right-sided; O, absent expression), with the exception of bilateral expression. Sagittal section electron micrograph (P) of the roof of Kupffer's vesicle showing, a single cilium and associated basal body. (Q) Diagram of the micrograph in P, detailing how the angle of the basal body [approximately 45░ to the posterior (P)] would result in the cilium projecting into Kupffer's vesicle on the right-to-left portion of the counterclockwise rotary beat. (R) Frequency of laterality defects in polaris and hippi morphant embryos. Expression of pitx2 and southpaw was randomized in polarisAUG embryos, while hippiSP embryos showed significantly higher numbers of embryos with no expression of southpaw and pitx2 (control, n=25; polarisAUG/pitx2, n=29; polarisAUG/southpaw, n=36; hippiSP/pitx2, n=40; hippiSP/southpaw, n=57). In embryos lacking laterality signals in the lateral plate mesoderm, gene expression was nevertheless maintained in the tailbud (spaw) and Rohon-Beard neurons (pitx2). Scale bar: 10 Ám. a, anterior; d, dorsal. EXPRESSION / LABELING:
|
|
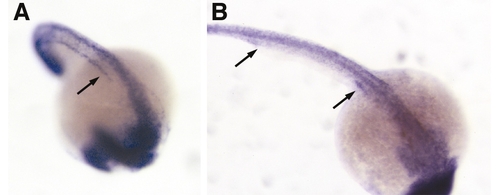
Ubiquitous expression of polaris and hippi in the developing zebrafish embryo. In situ expression pattern of polaris at 21-somite (A) and hippi at 24 hpf. (B). Both genes are ubiquitously expressed, with polaris expression enriched along the forming pronephric duct (arrow, A). (B) hippi expression can be seen in the anterior and posterior parts of the pronephric duct (arrows). |
|
Rescue of IFT morphant phenotype by hippi mRNA injection. Overexpression of zebrafish hippi by injection of capped RNA (2.3 ng) does not cause a visible phenotype, but is capable of rescuing the phenotype caused by injection of 4.6 nl of 0.1 mmol/l hippi SP morpholino. All 55 morpholino-injected embryos showed pronephric cyst formation (arrow and * in histological cross section) and some degree of hydrocephalus (arrowhead and ** in histological cross section), with 48 of 55 also having a ventrally bent body axis, whereas 6 appeared normal. Of the 44 co-injected embryos, 4 had a bent body axis, and hydrocephalus, but no renal cyst formation. Four had a straight body axis and no hydrocephalus, but kidney cysts, and 36 appeared wild type, which was confirmed by histology. |
|
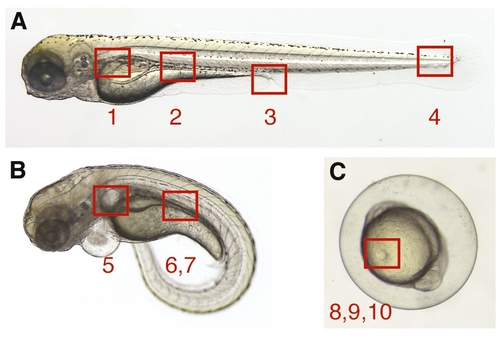
Topographical anatomy of supplemental quicktime movies. The numbered areas specify the anatomical region in the zebrafish embryo/larva examined by videomicroscopy in quicktime movies. (A) Wild-type embryo: (1) Movie 1; (2) Movie 2; (3) Movie 3; (4) Movie 4. (B) Morphant embryo: (5) Movie 5; (6) Movie 6; (7) Movie 7. (C) Wild-type embryo, 10-somite stage: (8) Movie 8; (9) Movie 9; (10) Movie 10. Movies 1, 2, 4, 5, 6, 7 and 8 are in slow motion (163); 9 and 10 are real-time; and 3 is in fast motion (153) |

Unillustrated author statements EXPRESSION / LABELING:
|