FIGURE
Fig. 1
- ID
- ZDB-FIG-190724-16
- Publication
- Seda et al., 2019 - An FDA-Approved Drug Screen for Compounds Influencing Craniofacial Skeletal Development and Craniosynostosis
- Other Figures
- All Figure Page
- Back to All Figure Page
Fig. 1
|
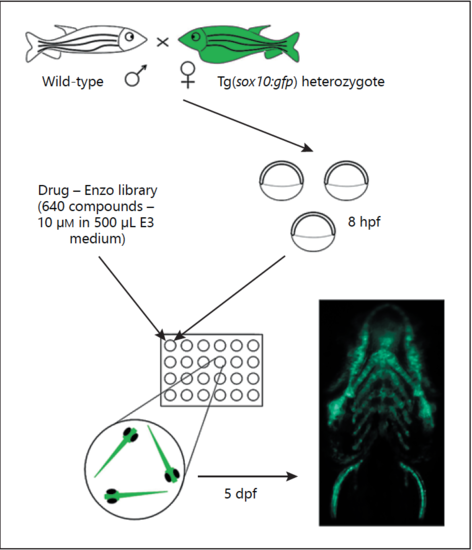
Overview of zebrafish drug screen employed in this study. Three embryos from a wild-type x sox10:gfp heterozygote cross per well were treated with one of 640 FDA-approved drugs in a 24-well plate format from 8 hours post-fertilisation (hpf) and screened for quantitative increases in the angle of the ceratohyoid cartilage or gross malformations at 5 days post-fertilisation (dpf). |
Expression Data
Expression Detail
Antibody Labeling
Phenotype Data
Phenotype Detail
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Mol. Syndromol.