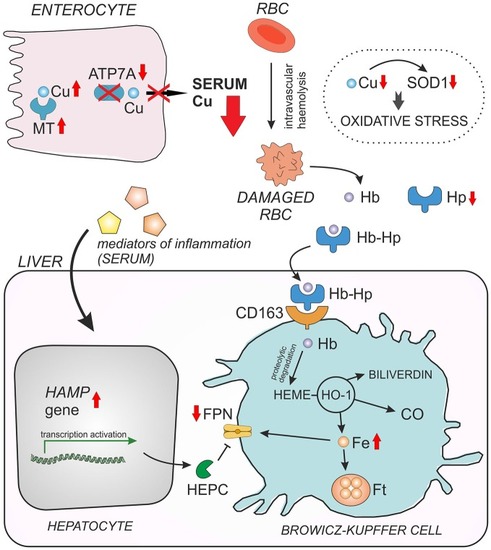
Interaction between copper (Cu) and iron (Fe) in young mosaic mice. Duodenal enterocytes can export copper across the basolateral membrane by ATP7A protein. Due to ATP7A gene mutation in mosaic mice, copper cannot be released to the serum and accumulates within the enterocytes in a complex with metallothionein (MT). Decreased serum Cu level entails Cu deficiency in red blood cells (RBC) and in consequence reduced activity/expression of Cu, Zn-superoxide dismutase (SOD1), which play a crucial role in RBC antioxidant defense. As the result, Cu-deficient RBC of mosaic mice display morphological abnormalities and undergo intravascular hemeolysis connected with hemoglobin (Hb) release to the serum and haptoglobin-dependent (Hp) elimination of free Hb from the circulation. When Hb is released from damaged RBC, it is instantly bound by haptoglobin (Hp) and forms a Hp–Hb high-affinity complex. This complex is then rapidly taken up from the circulation by the CD163 receptor present mainly on tissue macrophages (in the liver on Browicz-Kupffer cells). The CD163 receptor has no measurable affinity for free Hp. Thus, specific recognition of Hp–Hb by CD163 explains the decrease in Hp concentration in the serum during accelerated hemeolysis. The proteolytic Hb degradation in Browicz-Kupffer cells leads to the release of heme, which is then enzymatically decomposed by heme oxygenase 1 (HO-1) resulting in the formation of carbon monoxide (CO), biliverdin and Fe. Non-heme iron can be then stored as a complex with ferritin (Ft) or exported outside the cell by ferroportin (FPN), the sole cellular exporter of ionic iron known in mammalian cells. The content of hepatic non-heme Fe is elevated in mosaic mice, probably due to decreased expression of FPN. The concentration of cell surface Fpn largely depends on the post-translational regulation through internalization and degradation following hepcidin (Hepc) binding. Down-regulation of FPN expression in the liver of young mosaic mice is probably due to the concomitant up-regulation of hepatic hepcidin gene (Hamp), synthesized mainly in hepatocytes in response to systemic inflammation reported to occur in mosaic mice.
|