Fig. 6
|
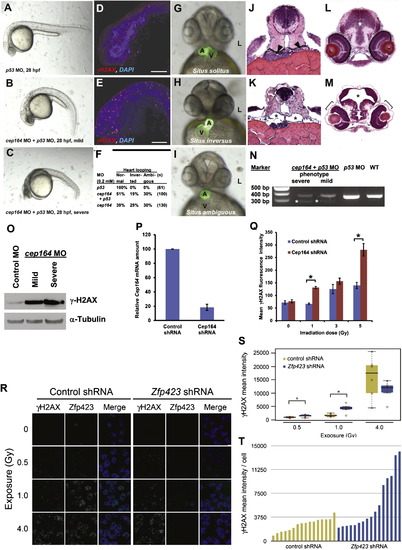
Knockdown of cep164 in Zebrafish Embryos Results in Ciliopathy Phenotypes, and Knockdown of Cep164 or Zfp423(Znf423) Causes Sensitivity to DNA Damage A morpholino-oligonucleotide (cep164 MO) targeting the exon 7 splice donor site of zebrafish cep164 was injected into fertilized eggs at the one to four-cells stage together with p53 MO (0.2 mM) to minimize nonspecific MO effects. (A–E) Whereas p53 MO injection (n = 67) did not produce any phenotype (A), coinjection of cep164 MO at 28 hpf caused the mild ciliopathy phenotype of ventral body axis curvature in 48% of embryos (60/125) (B). 50% of embryos (62/125) showed severe cell death throughout the body as judged by gray-appearing cells in the head region (C). Embryos with severe cell death also showed increased expression of phosphorylated γH2AX (D) compared to p53 MO control (E). Most embryos with massive cell death did not survive beyond 48 hpf. (F–I) At 48 hpf, surviving cep164 morphants displayed the ciliopathy phenotype of laterality defects. Whereas p53 MO did not cause any abnormal heart looping (F and G), cep164 MO caused inverted heart looping (H) or ambiguous heart looping (I). (A, atrium; L, left; V, ventricle). (J–M) At 72 hpf, cep164 morphant embryos developed further ciliopathy phenotypes. When compared to p53 MO controls (J), pronephric tubules (arrow heads) exhibited cystic dilation (K), asterisks) in 25% (7/28) of embryos, compared to p53 MO controls (J and L), 0% (0/67) of which showed kidney cysts, hydrocephalus (asterisk), or retinal dysplasia (brackets) (M). (N) At 0.2 mM, cep164 MO knockdown effectively altered mRNA processing as revealed by RT-PCR. The wild-type (WT) mRNA product is 339 bp. A shorter aberrantly spliced mRNA product appeared in cep164 morphants (asterisks), and the normal mRNA product was significantly reduced. p53 MO alone did not affect cep164 mRNA processing. (O) Quantification of γ-H2AX levels in cep164 MO morphants. Whole-fish lysates were prepared from morphants injected with control MO (p53 0.2 mM) or cep164 MO (p53 0.2 mM, cep164 0.2 mM). Injection of cep164-targeting MO causes upregulation of γ-H2AX in cep164 morphant embryos signifying perturbed DDR. γ-H2AX levels correlate with the phenotypic severity of the cep164 morphants (see A–C). Anti-α-tubulin antibody was used to show equal loading. (P and Q) Cep164-deficient IMCD3 cells exhibit radiation sensitivity. In IMCD3 cells transduced with shRNA retrovirus, Cep164 expression was suppressed by shRNA knockdown to about 20% of control as judged by qPCR (P). Cep164 knockdown resulted in a dose-dependent increase of γH2AX-positive cells in a FACS assay, signifying increased radiation sensitivity to IR and perturbed DDR. See also Figure S7. In (Q) the level of significance of two-tailed t test (p < 0.001) is indicated by an asterisk. Error bars denote SEM. (R–T) Zfp423(Znf423)-deficient P19 cells exhibit radiation sensitivity. P19 cells transduced with shRNA lentivirus were exposed to the indicated level X-irradiation. Zfp423 and γH2AX immunofluorescence was quantified in matched replicate cultures for each virus 2 hr after irradiation. (R) Representative images illustrate dose-responsiveness of γH2AX and effective knockdown of Zfp423 expression. (S) γH2AX intensity normalized to DAPI+ nuclei is increased following IR at 0.5 and 1.0 Gy, signifying increased IR sensitivity and perturbed DDR (2 fields from each of 6 replicate cultures per condition). Asterisks, uncorrected pair-wise p < 0.05, Mann-Whitney U test, 2 tails. (T) Histogram shows average γH2AX intensity per cell in 16 additional replicate cultures for each shRNA at 1.0 Gy exposure. p = 0.018, Mann-Whitney U test, 2 tails. See also Figure S7. Box plots delimit quartiles in (S). |
| Fish: | |
|---|---|
| Knockdown Reagents: | |
| Observed In: | |
| Stage Range: | Prim-5 to Protruding-mouth |
Reprinted from Cell, 150(3), Chaki, M., Airik, R., Ghosh, A.K., Giles, R.H., Chen, R., Slaats, G.G., Wang, H., Hurd, T.W., Zhou, W., Cluckey, A., Gee, H.Y., Ramaswami, G., Hong, C.J., Hamilton, B.A., Cervenka, I., Ganji, R.S., Bryja, V., Arts, H.H., van Reeuwijk, J., Oud, M.M., Letteboer, S.J., Roepman, R., Husson, H., Ibraghimov-Beskrovnaya, O., Yasunaga, T., Walz, G., Eley, L., Sayer, J.A., Schermer, B., Liebau, M.C., Benzing, T., Le Corre, S., Drummond, I., Janssen, S., Allen, S.J., Natarajan, S., O'Toole, J.F., Attanasio, M., Saunier, S., Antignac, C., Koenekoop, R.K., Ren, H., Lopez, I., Nayir, A., Stoetzel, C., Dollfus, H., Massoudi, R., Gleeson, J.G., Andreoli, S.P., Doherty, D.G., Lindstrad, A., Golzio, C., Katsanis, N., Pape, L., Abboud, E.B., Al-Rajhi, A.A., Lewis, R.A., Omran, H., Lee, E.Y., Wang, S., Sekiguchi, J.M., Saunders, R., Johnson, C.A., Garner, E., Vanselow, K., Andersen, J.S., Shlomai, J., Nurnberg, G., Nurnberg, P., Levy, S., Smogorzewska, A., Otto, E.A., and Hildebrandt, F., Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling, 533-548, Copyright (2012) with permission from Elsevier. Full text @ Cell