- Title
-
Zagociguat prevented stressor-induced neuromuscular dysfunction, improved mitochondrial physiology, and increased exercise capacity in diverse mitochondrial respiratory chain disease zebrafish models
- Authors
- Burg, L., Yoon, H., Peng, M., Germano, P., Reesey Gretzmacher, E., Xiao, R., Anderson, V.E., Nakamaru-Ogiso, E., Falk, M.J.
- Source
- Full text @ Front Pharmacol
|
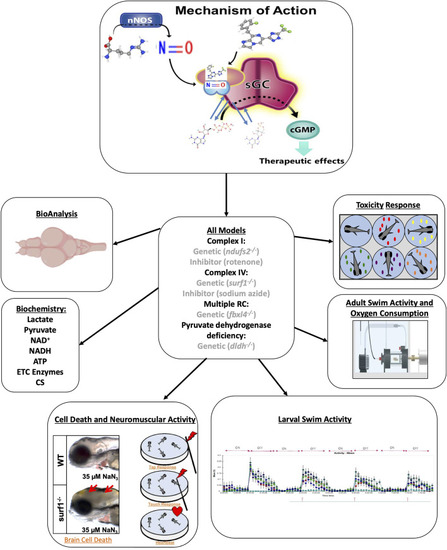
Evaluation of zag in multiple zebrafish models of mitochondrial dysfunction. Zag is a small-molecule sGC stimulator (structure at top) that increases NO-sGC-cGMP signaling. Here, we tested zag in multiple larval and adult zebrafish genetic and/or pharmacologic models of mitochondrial disease (center). Toxicity was evaluated in larval fish, and accumulation analysis (BioAnalysis) was performed on adult zebrafish brain and tail muscle. The larval models were evaluated for cell death, as evidenced by the presence of a gray brain and neuromuscular activity (lower left and upper right), swimming activity (lower right), and classical biochemical markers (middle left). The adult models were evaluated in swim activity assays (middle right, Loligo swim tunnels) while measuring oxygen consumption during a defined exercise protocol. |
|
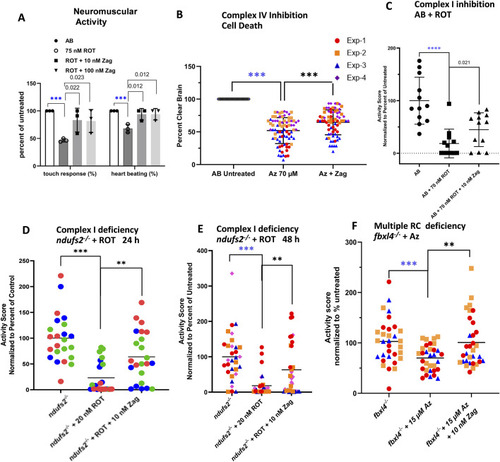
Zag rescues neuromuscular deficits in pharmacological models of complex I and complex IV deficiency. |
|
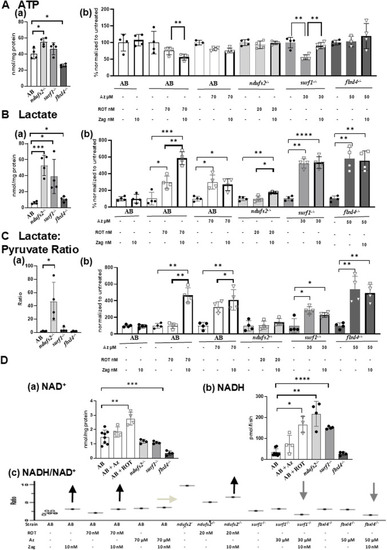
Effects of zag treatment on ATP, lactate, lactate/pyruvate, and NADH/NAD levels in pharmacological and genetic models of complex I and complex IV deficiency. |
|
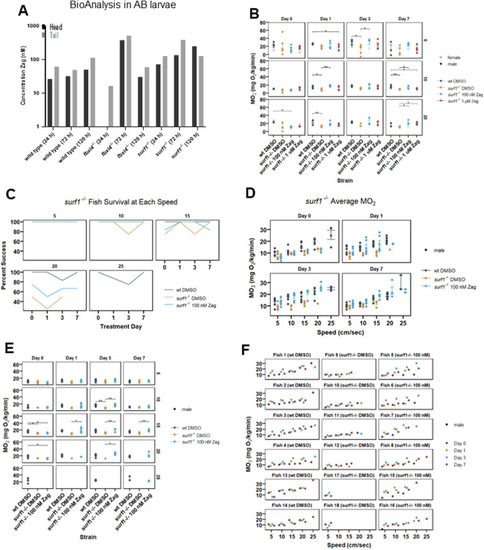
Zag accumulates in adult zebrafish brain and tail muscle while affecting adult swim capacity and oxygen consumption rate in |