- Title
-
Trimetazidine stimulates intracellular Ca2+ transients and zebrafish locomotor activity in spinal neurons
- Authors
- Bernardi, S., Vitolo, S., Gabellini, C., Marchese, M., Ferraro, E.
- Source
- Full text @ Sci. Rep.
|
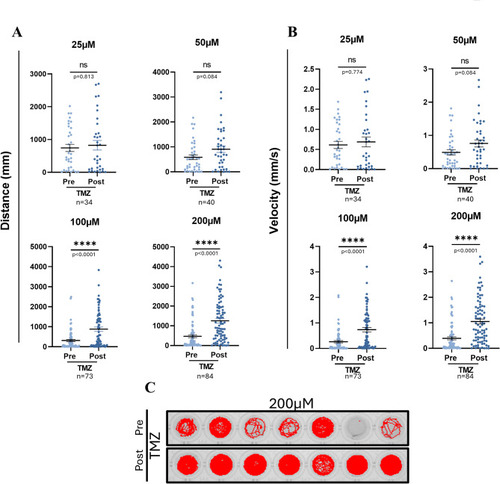
Locomotor behavior in TMZ-treated zebrafish larvae. ( |
|
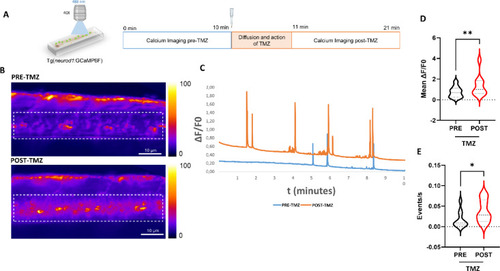
Spinal cord Ca2+ imaging upon TMZ exposure. ( |
|
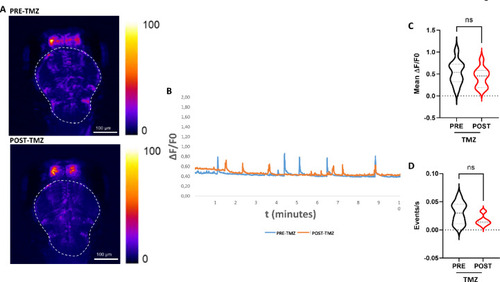
Whole brain Ca2+ imaging upon TMZ exposure. ( |
|
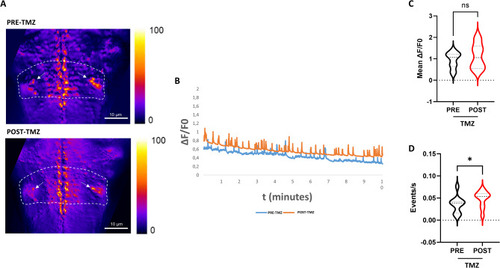
Hindbrain Ca2+ imaging upon TMZ exposure. ( |