- Title
-
Loss of factor VIII in zebrafish rebalances antithrombin deficiency but has a limited bleeding diathesis
- Authors
- Azhwar, R., Richter, C.E., Griffin, M.S., Emly, S.M., Yaman, M., Arruda, V.R., Samelson-Jones, B.J., Shavit, J.A.
- Source
- Full text @ Blood Adv
|
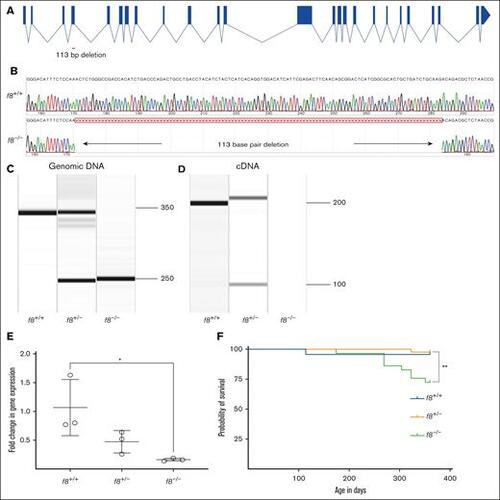
Targeting of the f8 locus produces a null allele. (A) Structure of the f8 zebrafish gene and location of the exon 4 deletion. (B) Sequencing of f8–/– cDNA demonstrates a 113 bp deletion resulting in a frameshift mutation with a premature stop codon. Amplification of (C) genomic DNA and (D) cDNA from f8+/− incrosses. (E) RT-qPCR performed in f8+/− incrosses; f8–/– fish show a significant decrease of 90% (± 28%) expression levels compared to f8+/+. The mean cycle threshold (Ct) values were compared to the reference gene β-actin to calculate the relative fold change. Error bars represent standard deviation, and statistical significance was determined by a Student t test (P = .03). (F) Survival curves of zebrafish offspring from an f8 heterozygous incross starting at 3 months of age shows a statistically significant loss of 25% of homozygotes by 1 year of age (∗∗P < .002 by log-rank Mantel-Cox testing), f8+/+ n = 23, f8+/− n = 42, f8–/– n = 29. ∗, P < .05. |
|
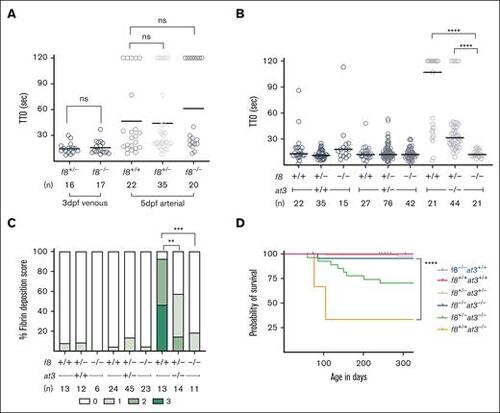
f8 mutants show an altered hemostatic balance compared to mammals. (A) FVIII-deficient zebrafish laser-mediated endothelial injury of the PCV was performed on larvae at 3 dpf, and dorsal aorta at 5 dpf. The TTO was not significantly different in f8–/– compared to f8+/− clutchmates (Mann-Whitney U test P > 1). Circles represent individual larvae. Horizontal bars represent the median TTO. (B) Laser-mediated injury on f8+/−;at3+/− incrosses reveal that loss of FVIII reverses the at3–/– phenotype. In the at3–/– background, the increased TTO is reversed by mutation of f8 in a dose-dependent fashion (∗∗∗∗P < .001 by Mann-Whitney U testing). (C) Fibrin deposition observed in 5 dpf fgb-egfp larvae resulting from f8+/−;at3+/− incrosses. Bar graph represents the percentage of larvae in each fibrin deposition category: score 0 having no GFP-labeled fibrin deposits in the PCV, score 1 with <5 occurrences, score 2 with 5 to 25 occurrences, and score of 3 with widespread continuous threads of fibrin in the PCV and/or surrounding regions. Overall statistical significance was determined by Kruskal-Wallis and pairwise comparisons among the f8+/+;at3–/–, f8+/−;at3–/–, and f8–/–;at3–/– mean fibrin scores by a Wilcoxon rank sum test with a Bonferroni correction (∗∗P < .005; ∗∗∗P < .0005). (D) Survival curve of zebrafish offspring from f8+/−;at3+/− incrosses shows that loss of f8 rescues the at3–/– lethal phenotype with a statistically significant difference between f8+/+;at3–/– and f8–/–;at3–/– (∗∗∗∗P < .0001 by log-rank Mantel-Cox testing). f8+/+;at3+/+ n = 22, f8+/−;at3+/− n = 83, f8+/+;at3–/– n = 3, f8+/−;at3–/–, n = 27, f8–/–;at3–/–, n = 22, f8–/–;at3+/+ n = 18. ns, nonsignificant. |
|
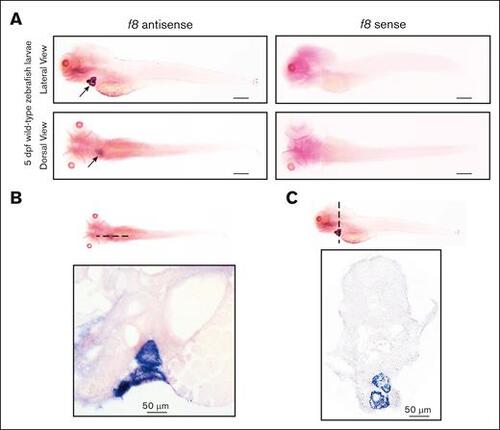
WISH of f8 expression in wild-type zebrafish larvae at 5 dpf. (A) In situ hybridization with an antisense probe shows f8 expression in the heart, while a sense control probe shows no signal. Scale bars, 500 μm. f8 expression is located in the atrial and ventricular walls of the heart as shown in (B) sagittal and (C) transverse sections (10 μm) of stained larvae. |
|
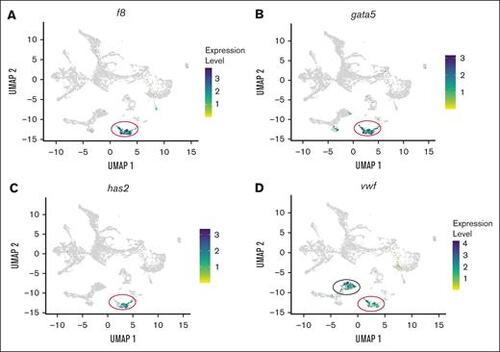
scRNA-seq analysis of f8 and vwf expression in endothelial cells isolated from kdrl-egfp transgenic zebrafish larvae. (A) UMAP (uniform manifold approximation and projection) projection scRNA-seq data from endothelial cells showing f8 expression localized specifically to the endocardial cluster, marked by a red circle. (B-C) Colocalization analysis of f8 with endocardial endothelial–specific markers gata5 and has2 confirms endothelial-specific expression of f8. The red circle marks the endocardial cluster. (D) Expression pattern of vwf, demonstrating localization in both the endocardial (red circle) and arterial endothelial (black circle) clusters. |
|
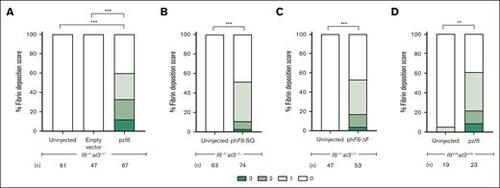
Overexpression of FVIII results in thrombosis. Either f8+/+;at3+/+ or f8–/–;at3–/– zebrafish, both in the fgb-gfp background, were incrossed and 1-cell stage embryos injected with pzf8, phF8-SQ, or phF8-ΔF and evaluated for spontaneous thrombi formation via fibrin deposition scoring at 5 dpf. pzf8 injected into (A) f8–/–;at3–/– and (D) f8+/+;at3+/+ zebrafish larvae exhibited statistically significant spontaneous thrombus formation. Empty vector demonstrates the baseline thrombus formation in f8–/–;at3–/– zebrafish larvae without functional FVIII activity. (B) phF8-SQ and (C) phF8-ΔF injected into f8–/–;at3–/– zebrafish larvae demonstrated statistically significant levels of spontaneous thrombi. Statistical significance was determined by ordinal logistic regression followed by Dunnett adjustments (∗∗∗P < .001; ∗∗P < .01). |
|
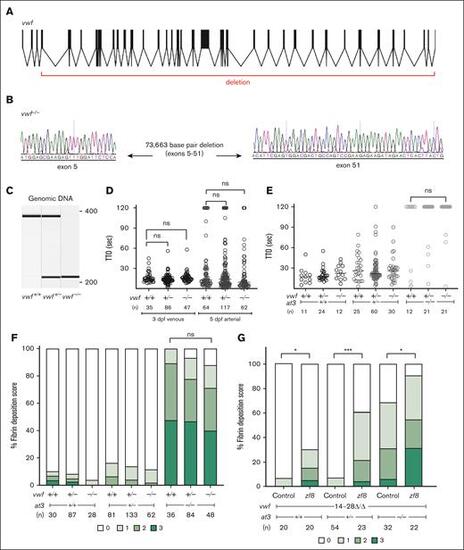
Genome engineering of a large deletion in vwf. (A) The structure of the vwf gene in zebrafish, with deletion indicated. (B) Sequencing of vwf–/– fish shows a large deletion (73.7 kb) between exons 5 and 51. (C) Genomic DNA amplification of vwf mutants. (D) Laser-mediated endothelial injury was performed on larvae at 3 dpf (venous) and 5 dpf (arterial). The TTO was not significantly different among vwf mutant clutchmates (Mann-Whitney U, P = .5 for venous and P = .04 for arterial, only values <.03 are considered significant after Bonferroni correction). Circles represent individual larvae. Horizontal bars represent the median TTO. (E) Laser-mediated injury at 3 dpf from an incross of vwf+/−;at3+/− shows no change in the at3–/– DIC bleeding phenotype with loss of Vwf (Mann-Whitney U, P = .12). (F) Spontaneous fibrin deposition observed in 5 dpf larvae from vwf+/−;at3+/− incrosses. Bar graph represents percentage of larvae in each category: score 0 having no GFP-labeled fibrin deposits in the PCV, score 1 <5 occurrences, score 2 with 5 to 25 occurrences, and score 3 with widespread continuous threads of fibrin in the PCV and/or surrounding regions. Statistical significance was determined by Kruskal-Wallis testing and pairwise comparisons among vwf+/+;at3–/–, vwf+/−;at3–/–, and vwf–/–;at3–/– (P = .3). Larvae were confirmed to express the fgb-egfp transgene in the liver prior to scoring. (G) pzf8 injected into 1-cell stage embryos collected from vwf14-28Δ/Δ;at3+/− incrosses and evaluated for spontaneous thrombi formation via fibrin deposition scoring at 5 dpf. pzf8 injected zebrafish larvae exhibited significant fibrin deposition compared to uninjected controls in all genotypes. Statistical significance was determined by ordinal logistic regression followed by Dunnett adjustments (∗∗∗P < .001; ∗P < .05). ns, nonsignificant. |
|
Cross-species rescue of fibrin deposition in f8–/–;at3–/– larvae by FVIII and VWF. (A) Model demonstrating the effect of FVIII levels on fibrin deposition. (B) f8–/–;at3–/– zebrafish larvae were injected with various combinations of plasmids encoding human (h) and zebrafish (z) FVIII and VWF and analyzed for fibrin deposition at 5 dpf by an observer blinded to condition. Injection groups included: uninjected controls, hVWF, hF8, zvwf, zf8, coinjection of hVWF + hF8, coinjection of zvwf + zf8, and cross-species combinations (hVWF + zf8 and zvwf + hF8). (C) Comparison of various groups shows that hVWF, but not zvwf, enhances fibrin deposition in combination with human or fish FVIII. Statistical analysis was performed using ordinal logistic regression followed by Dunnett's adjustments. (∗P < .05, ∗∗P < .01, ∗∗∗P < .001). ns, nonsignificant. |