- Title
-
Faberidilactone A, a Sesquiterpene Dimer, Inhibits Hepatocellular Carcinoma Progression Through Apoptosis, Ferroptosis, and Anti-Metastatic Mechanisms
- Authors
- Cao, R., Liu, Y., Bao, J., Rong, M., Xu, J., Liao, H., Guo, Y.
- Source
- Full text @ Molecules
|
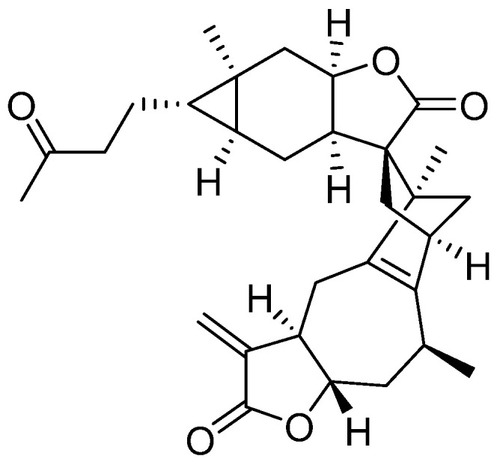
Chemical structure of faberidilactone A. |
|
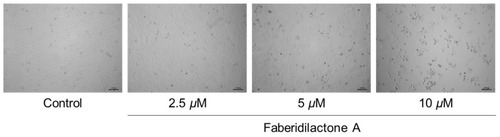
Faberidilactone A inhibits the proliferation of HepG2 cells. HepG2 cells were treated with faberidilactone A (2.5, 5, and 10 µM) for 48 h. Morphological changes in faberidilactone A-treated HepG2 cells were observed with a microscope, bar: 100 µm. |
|
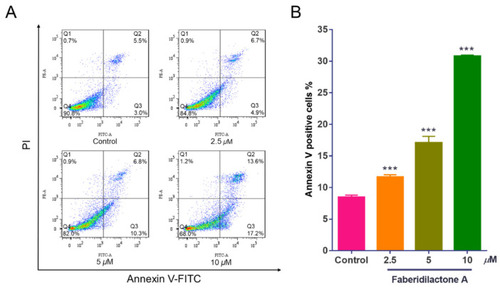
Faberidilactone A induces apoptosis in HepG2 cells. HepG2 cells were treated with different concentrations (2.5, 5, and 10 µM) of faberidilactone A for 48 h, stained with Annexin V-FITC and PI, and then the proportion of apoptotic cells was detected by flow cytometry. ( |
|
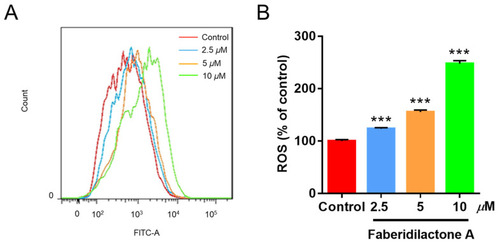
Faberidilactone A induces the accumulation of ROS in HepG2 cells. Cells were treated with different concentrations (2.5, 5, and 10 µM) of faberidilactone A for 48 h, stained with DCFH-DA, and analyzed by flow cytometry. ( |
|
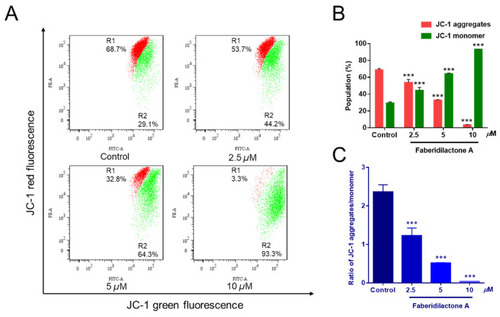
Faberidilactone A caused a decrease in MMP in HepG2 cells. Cells were treated with faberidilactone A for 48 h, stained with JC-1, and the red and green fluorescence intensity changes were detected by flow cytometry. ( |
|
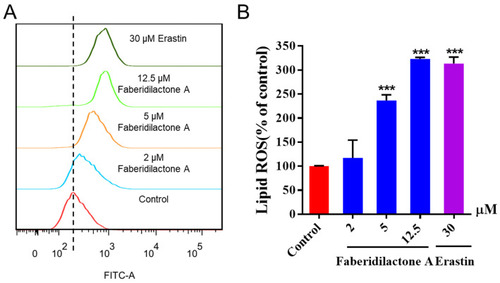
Faberidilactone A enhanced lipid ROS levels in HepG2 cells. After 48 h of treatment with different concentrations (2, 5, and 12.5 µM), cells were stained with C11 BODIPY 581/591 and analyzed by flow cytometry. ( |
|
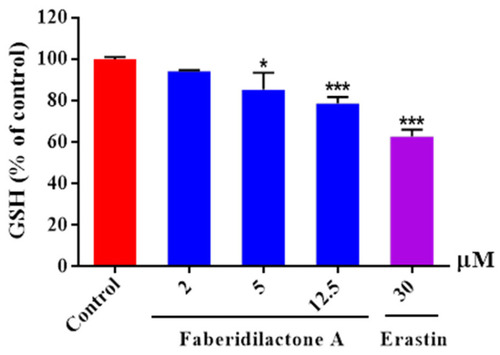
Faberidilactone A enhanced the depletion of GSH in HepG2 cells. Cells were lysed following a 24 h treatment with the drug at concentrations of 2, 5, and 12.5 µM. The absorbance of the resulting product was measured using a microplate reader, in accordance with the kit instructions. A quantitative analysis of the relative levels of GSH was performed, and all values are expressed as mean ± SD. * |
|
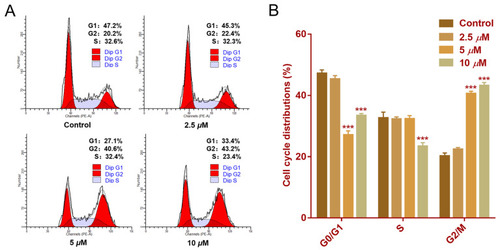
Effect of faberidilactone A on the cell cycle distribution of HepG2. HepG2 cells were treated with different concentrations (2.5, 5, and 10 µM) of faberidilactone A for 48 h. ( |
|
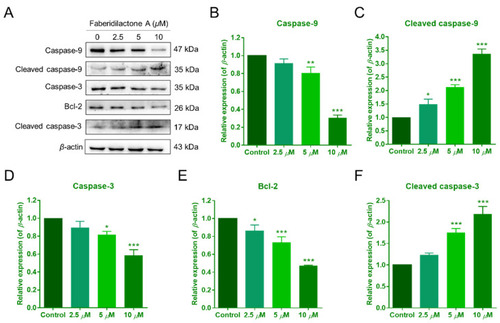
Faberidilactone A regulated the expression of apoptosis-related proteins. ( |
|
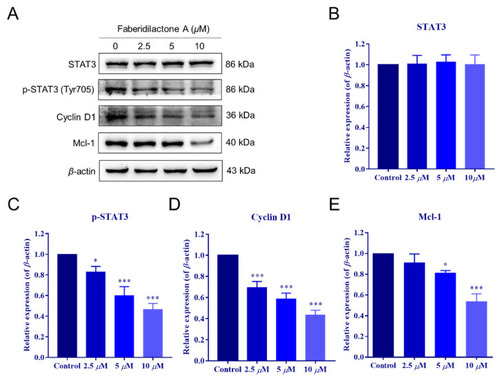
Faberidilactone A regulated the STAT3 signaling pathway. ( |
|
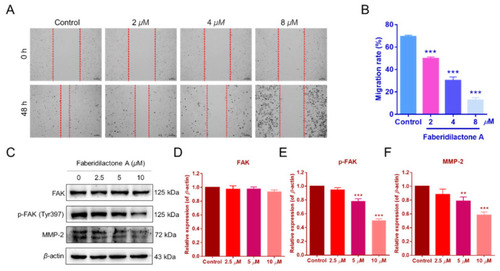
Faberidilactone A inhibited the migration of HepG2 cells and regulated the FAK signaling pathway. ( |
|
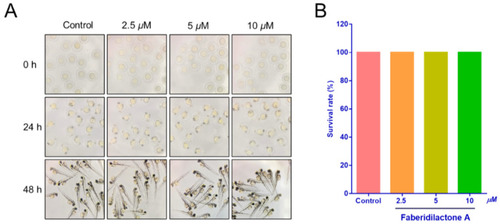
Toxicity of faberidilactone A to normal zebrafish embryos. ( |
|
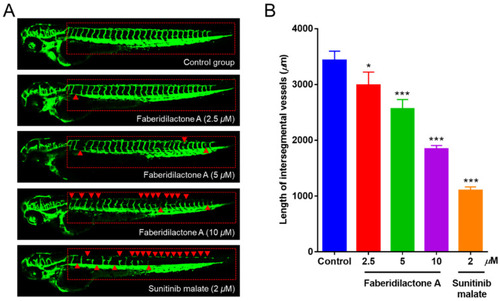
Faberidilactone A could inhibit angiogenesis in a transgenic zebrafish model. ( |
|
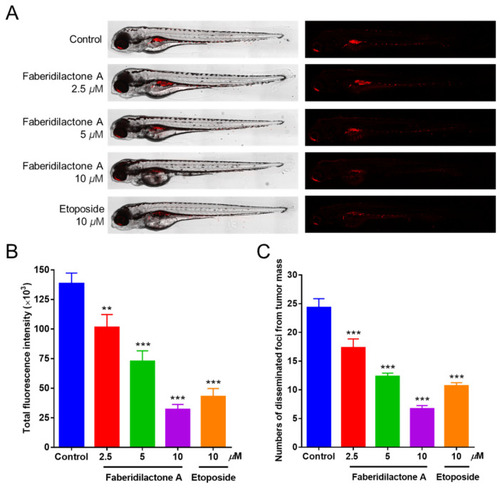
Antitumor effect of faberidilactone A in a zebrafish xenograft model. ( |