- Title
-
The piRNA protein Asz1 is essential for germ cell and gonad development in zebrafish and exhibits differential necessities in distinct types of germ granules
- Authors
- Ahmad, A., Bogoch, Y., Shvaizer, G., Guler, N., Levy, K., Elkouby, Y.M.
- Source
- Full text @ PLoS Genet.
|
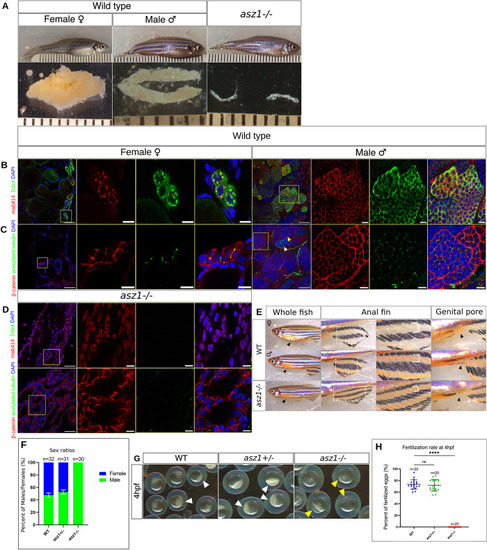
Loss of Asz1 results in germ cell loss and underdeveloped gonads and |
|
Asz1 is essential for germ cell and gonad development in juvenile post-embryonic stages. |
|
Asz1 is essential for germ cell survival during early gonad development. A. |
|
Zygotic Asz1 is not required for PGC specification and migration. |
|
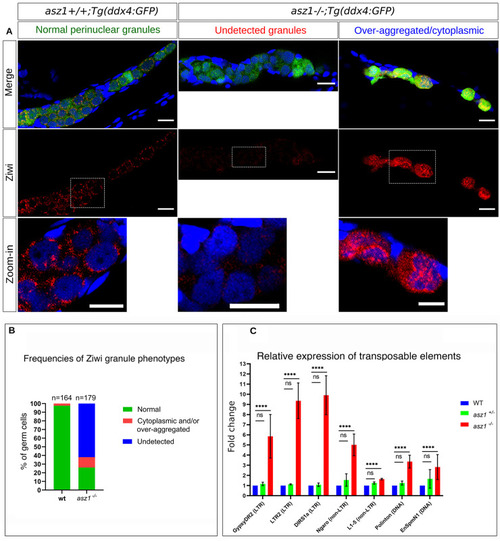
Asz1 is essential for piRNA granule organization ad repression of transposon expression. |
|
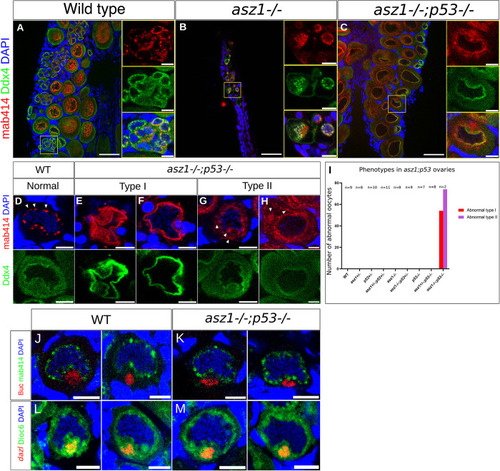
Partially rescued |
|
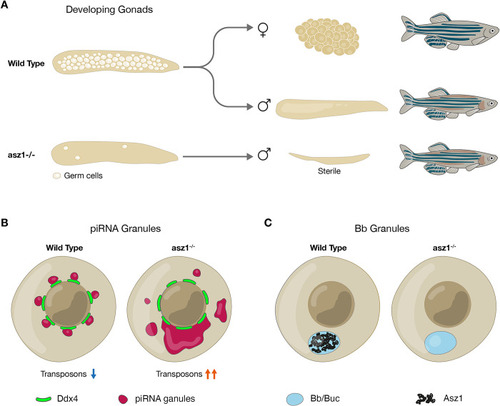
Schematics of the germline developmental requirement for Asz1 functions. |