- Title
-
Resveratrol alleviates heart failure by activating foxo3a to counteract oxidative stress and apoptosis
- Authors
- Liu, K., Zhu, Y., Gao, W., Han, X., Zhang, Q., Zhao, Y., Zu, Y.
- Source
- Full text @ Biomed. Pharmacother.
|
High-throughput screening reveals that resveratrol alleviates heart failure in zebrafish. (A) Schematic of the drug screening process. (B) Fluorescence microscopy analysis of zebrafish heart metrics. (C) High-throughput drug screening based on the cMAP database using zebrafish. (D) Bar plot shows the screening of 28 drugs based on the indicator of cardiac output (CO). The red line represents the mean CO of HF group (= 104) and the red dots stand for the mean CO of each drug group. (E) Bar plot shows the screening of 28 drugs based on the indicator of stroke volume (SV). The red line represents the mean SV of HF group (= 1.06) and the red dots stand for the mean SV of each drug group. The bars marked in green indicate RES groups. (F) Heatmap showing the specific metrics for high-throughput drug screening using zebrafish. (G) Two-dimensional and three-dimensional structures of resveratrol. |
|
RES has a mitigating effect on VPH-induced HF in zebrafish. (A) Hearts of zebrafish under bright field condition (left panel); confocal microscopy (middle panel); top view with fluorescence (cmlc2:EGFP) superimposed on bright field (right panel). (B) Under 80 μM VPH exposure, the pericardial cavity area of zebrafish in the 40 μM RES group was significantly reduced relative to the heart failure group. (C) Under 80 μM VPH exposure, the angle of cardiac looping in zebrafish in the 40 μM RES group tended to normalise compared to the heart failure group. (D) Under 80 μM VPH exposure, there was a significant recovery of zebrafish blood flow rate in the 40 μM RES group relative to the heart failure group. (Compared with the control group: *: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
Zebrafish cardiac output, heart rate, and behavioural acquisition. (A) Relief of heart failure in zebrafish observed by ventricular filling, ventricular ejection, and isovolumic diastole conditions. The location of heart is shown as a dotted line. (B) Changes in the dynamic gray values of the zebrafish heart. (C) Under 80 μM VPH exposure, heart rate was significantly increased in the 40 μM RES group compared to the VPH modeling group. (D) Under 80 μM VPH exposure, heart output was significantly restored in the 40 μM RES group compared to the VPH modeling group. (E) Behavioural tracks (left) and heatmap (right) of trajectories. (F) Zebrafish behavioural distance analysis. (Compared with the control group: *: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
Resveratrol mitigation of mitochondrial membrane potential disorder and oxidation experiment. (A) The remission of heart failure in zebrafish was observed by the heat map distribution of calcium signal activation time. The cardiac calcium signals of CaD90 (B), CaD30/80 (C), and CaD dispersion (D) in the RES exposure group were significantly decreased compared with those in the VPH group. (E) In the AC16 cell mitochondrial assay, it can be observed that the mitochondrial damage of AC16 cells was alleviated under the co-exposed of VPH and RES. (F) Reactive oxygen species assay, where a reduction in the degree of oxidative damage in zebrafish is observed when VPH and RES co-exposed. (G) AC16 cell JC-1 aggregate area. (H) AC16 cell JC-1 monomer area. (I) Reactive oxygen species assay with fluorescence intensity statistics. (Compared with the VPH group: *: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
Zebrafish cardiac cell apoptosis assay with AC16 cells to validate RES mitogenic effect. (A) Zebrafish tunnel staining assay. (B) Tol2 EGFP fluorescent plasmid transfection of AC16 cells. (C) VPH on AC16 cells viability assay. (D) RES on AC16 cells viability assay. (E) VPH combined with RES administration assay to detect the remission effect of RES on cells assay. (*: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
qPCR experiments and in situ hybridization experiments revealed RES regulating genes. (A) Results of qPCR experiments on sirt1, nppb, nppa, foxo1a, foxo1b, and foxo3a. (B) In situ hybridization maps of nppb and nppa. (C) In situ hybridization maps of foxo1b and foxo3a.(*: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
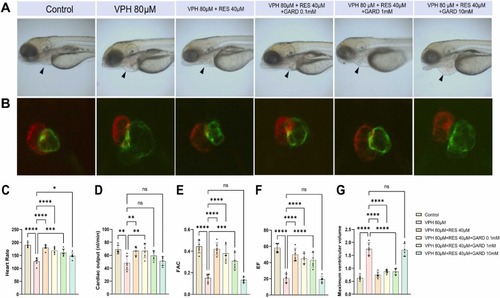
The effect of Gardenoside on heart failure in zebrafish. (A) Gardenoside inhibited the remission of heart failure in zebrafish and showed a dose-dependent effect. (B) Fluorescence images of ventral view of zebrafish heart under the effect of Gardenoside. (C) Effect of Gardenoside on zebrafish heart rate. (D) Effect of Gardenoside on zebrafish cardiac output. (E) Effect of Gardenoside on fractional area change in zebrafish. (F) Effect of Gardenoside on ejection fraction in zebrafish. (G) Effect of Gardenoside on the maximum ventricular area in zebrafish. (*: P < 0.05, **: P < 0.05, ***: P < 0.001, ****: P < 0.0001, NS: not statistically different). |
|
Schematic diagram of the mechanisms by which resveratrol alleviates heart failure. (A) Study design. (B) Celluar mechanism of resveratrol attenuates oxidative stress and apoptosis. (C, D) Molecular mechanism of resveratrol alleviates heart failure. |