- Title
-
Human atherosclerotic plaque transcriptomics reveals endothelial beta-2 spectrin as a potential regulator a leaky plaque microvasculature phenotype
- Authors
- Rademakers, T., Manca, M., Jin, H., Orban, T., Perisic, L.M., Frissen, H.J.M., Rühle, F., Hautvast, P., van Rijssel, J., van Kuijk, K., Mees, B.M.E., Peutz-Kootstra, C.J., Heeneman, S., Daemen, M.J.A.P., Pasterkamp, G., Stoll, M., van Zandvoort, M.A.M.J., Hedin, U., Dequiedt, F., van Buul, J.D., Sluimer, J.C., Biessen, E.A.L.
- Source
- Full text @ Angiogenesis
|
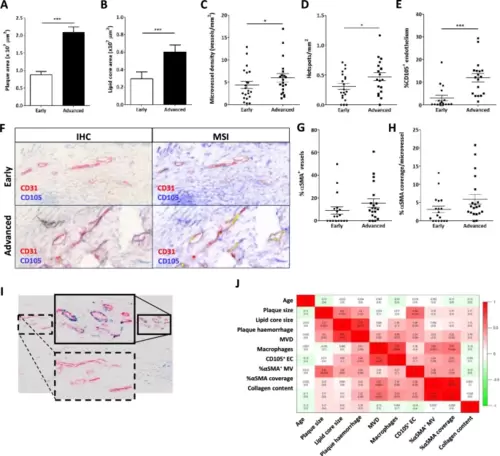
Intraplaque microvessel characteristics differ between advanced stable and advanced unstable atherosclerotic lesions. A + B Plaque (A) and lipid core (B) size of the Maastricht Human Plaque Study cohort were determined and were, as expected, significantly increased in advanced lesions with hemorrhage compared to the stable early lesions. C + D Concomitantly, there was an increase in both microvessel density (C) and the number of microvascular hotspots (D) within the advanced lesions. (E + F) The percentage of angiogenic endothelial cells (CD105 + CD31+) was determined by double staining for CD105 and CD31 and subsequent multispectral imaging (MSI) and unmixing of the CD105 and CD31 signals. Image analysis showed that the proportion of CD31 + endothelial cells that were also positive for CD105 + (angiogenic EC) was significantly increased in advanced versus early plaques (E). Representative images of the double staining are presented in panel F. G + H Stability of the microvessels, assessed by percentage of vessels covered by αSMA+ smooth muscle cells (SMC, G), as well as the amount of SMC coverage per vessel (percentage αSMA-positivity, H) were not significantly different. I This lack of effect may be, in part, caused by the high variability in the degree of αSMA-coverage between different CD31+ hotspots. J We did not observe significant correlations between MVD and angiogenesis (CD105+) or vessel maturation αSMA+. Data are from 22 patients (both stable and unstable advanced lesions) (A–J); error bars indicate SEM (A–E, G, H); *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005. Scalebars indicate 20µm |
|
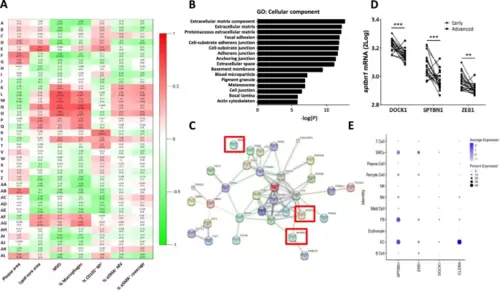
Microarray analysis shows clear correlation of MVD with specific modules, which are enriched for GO terms related to endothelial junctions and cytoskeletal organization. A Heatmap of the module-trait correlations, showing upregulated (red) or downregulated (green) gene modules in relation to phenotypic traits in advanced unstable lesions. A high overlap in genetic profile was found for microvessel density (MVD) and lesional macrophage content (% Macrophages), while the percentage angiogenic endothelium (% CD105+ MV) showed a different expression profile. αSMA parameters showed certain overlap with MVD but failed to show highly enriched modules. Values of the Pearson's r coefficient and associated p-values (in parenthesis) are reported. B GO analysis on the overrepresented module shows a clear enrichment of biological processes related to endothelial cell adhesion, endothelial junctions, and extracellular matrix and cytoskeletal organization. C PPI network reconstruction for the most central and interconnected gene members of the MVD correlated module L revealed a network which included several known endothelial key proteins. Highlighted are the three hub genes showing the highest ranks based on ranking analysis, which were selected for further study: zeb1, sptbn1 and dock1 (enboxed). D Interrogation of single cell RNASEq dataset of Alsaigh et al. [20] revealed preferential expression of sptbn1 and to a lesser extent zeb1 and dock1 by plaque endothelial cells. Average expression is color coded (white to blue), node size reflects % of cells with target gene expression. The profile of the endothelial signature gene cldn5 profile is added to serve as reference |
|
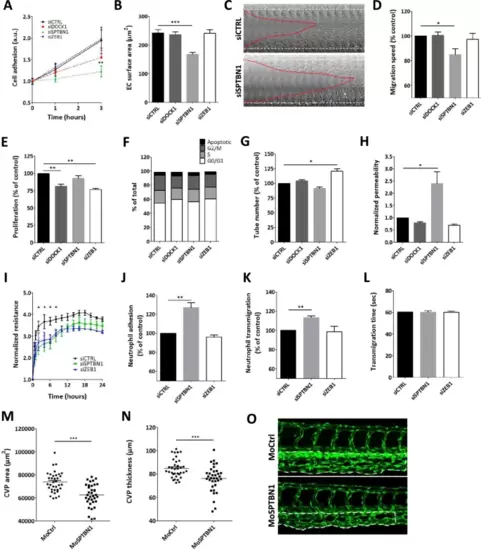
Knockdown of dock1, sptbn1, and zeb1 reveal a range of functional effects. A Endothelial cell adhesion was significantly altered for sptbn1 knockdown, whereas dock1 knockdown showed an intermediate phenotype and siZEB1 was ineffective. B For sptbn1, the reduced cell adhesion is accompanied by defective cell spreading. C + D In addition, sptbn1 knockdown also reduced endothelial migration speed in a wound healing assay (C: example; D quantitative data). E Endothelial proliferation rate was impaired after dock1 and zeb1, but not sptbn1 knockdown; F cycle stage analysis did not reveal significant shifts in cell cycle stage. G Tube formation was slightly increased upon zeb1 knockdown, with a trend towards reduced tube formation in sptbn1 knockdown cells. H As a functional parameter, we assessed permeability for 150kD dextran in a Transwell system, revealing a significant increase in permeability upon knockdown of sptbn1, whereas dock1 or zeb1 silencing was ineffective. I In keeping, we observed decreased resistance of the endothelial monolayer upon sptbn11 but not zeb1 knockdown. J–L Furthermore, sptbn1 but not zeb1 knockdown also increased adhesion (J) and transmigration (K) of neutrophils under flow, while overall transmigration time (L) was unaffected. Based on these findings SNTBN1 was taken for in vivo validation in zebrafish. M, O Microinjection of zebrafish embryo’s with morpholino antisense oligonucleotides against sptbn1 led to a significant reduction in caudal vein plexus (CVP) development in zebrafish, as shown by the CVP area (M) and CVP thickness (N). Representative images of control and sptbn1 in zebrafish show de reduced CVP area and thickness (O, dotted area). Data are from three (A–G, I–L) or four (H) independent experiments or 35 zebrafish per group (M–O); error bars indicate SEM (A-C, E-N); *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 |
|
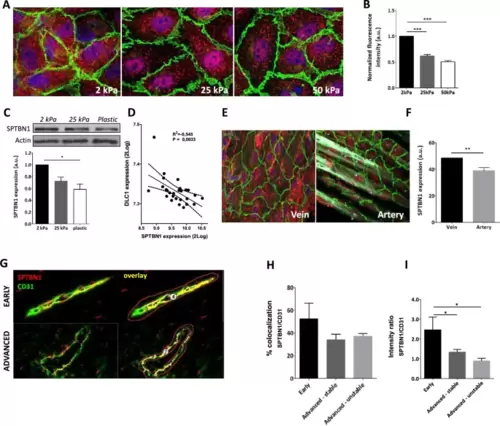
Increased stiffness reduced SPTBN1 protein expression both in vitro and in vivo. A HUVEC cultured on different stiffness gels showed reduced SPTBN1 protein expression by immunofluorescent staining (red). B Quantification of the IF staining showed a clear decrease in expression of SPTBN1 protein with increasing stiffness. C A similar stiffness associated reduction in SPTBN1 protein expression by endothelial cells was observed after Western Blot. D To confirm that SPTBN1 levels are stiffness dependent, the SPTBN1 expression was correlated to the stiffness sensitive marker DLC1, clearly showing reduced SPTBN1 expression upon higher stiffness. E + F To determine whether reduced SPTBN1 expression upon lower stiffness is relevant in vivo, we assessed mesenteric vein and artery from the same patient for SPTBN1 protein expression by immunofluorescence staining; a representative image is presented in E. We found that the softer vein had significantly higher endothelial expression of SPTBN1 compared to the stiffer artery (F). G–I Multispectral imaging of endothelial cells double stained for CD31 (green) and SPTBN1 (red) (G for representative images, costaining is given in yellow). Image analysis revealed a slight but not significant reduction in the level of colocalization of CD31 and SPTBN1 in microvessels in advanced vs early lesions (H), whereas relative SPTBN1 expression in plaque microvessels was significantly and progressively reduced with disease progression (I). Data are from three (A–D) or four (E, F) independent experiments or from 20 patient samples (G–I); error bars indicate SEM (B, C, D, F, H, I); *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005. Scalebars indicate 10µm (A and E), or 20µm (G) |
|
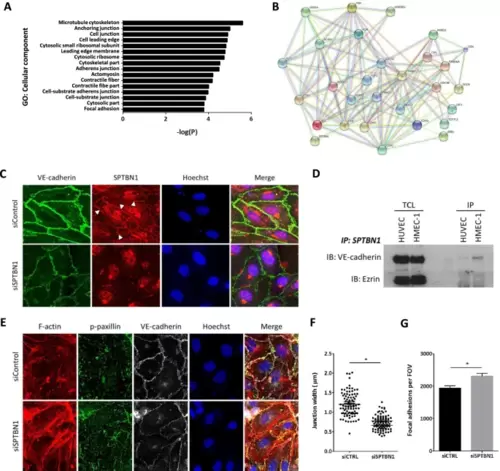
SPTBN1 has a potential role in cell junction regulation, is associated with VE-cadherin at endothelial cell junctions and is linked to focal adhesion regulation. A Sptbn1 was silenced in HMEC-1, after which gene expression was measured by microarray analysis. GO analysis of the differentially expressed genes revealed links to regulation of adherens junctions and cell junctions, as well as focal adhesions. B Subsequent network prediction analysis showed clear involvement of sptbn1, but also confirmed its connection with dock1 and zeb1 in these pathways, and its link to junctional proteins like VE-cadherin (CDH5), focal adhesion proteins like paxillin (PXN), and cell–matrix related proteins like vinculin (VCL). C Immunofluorescent staining of HMEC1 with silenced sptbn1 (or siControl treated controls) for SPTBN1 (red) and the endothelial junction protein VE-Cadherin (green); nuclei were counterstained with Hoechst 33,342. As expected, silencing led to sharply reduced SPTBN1 expression, while interestingly also VE-Cadherin expression was reduced. The latter was seen to be more dispersed over the whole cell after sptbn1 silencing, but is enriched at stable, straight junctions between cells, as well as at the basolateral side. At junctions, SPTBN1 was seen to colocalize with VE-cadherin. D Immuno-precipitation analysis of HUVEC and HMEC-1 for SPTBN1, followed by immunoblotting for VE-cadherin and Ezrin (loading control) demonstrated direct interaction of SPTBN1 and VE-Cadherin for both cell types. TLC: Total cell lysate, IP is Immuno-precipitate (E) Knockdown of SPTBN1 led to an increase in stress fibers and an increased staining of p-paxillin at the basolateral side of the cell, indicative of an increase in the number of focal adhesions, and in all, an altered cell adhesion to the substrate (F + G) Quantitative image analysis revealed reduced junctional width after sptbn1 silencing (F), with a concomitant increase in paxillin and focal adhesions (p-paxillin) at the basolateral side of the cell (G). Data are representative images from three independent experiments (C–G); error bars indicate SEM (F, G); * p ≤ 0.05. Scalebars indicate 10µm. D, F, H, I); *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005 |
|
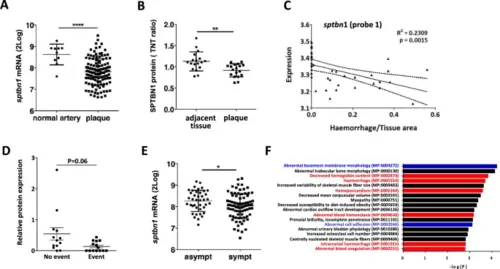
Validation of the association of SPTBN1 expression in human plaque and clinically relevant endpoints. A + B Validation of SPTBN1 expression in carotid artery plaque at mRNA (A) and protein level in the BIKE cohort (B), showing significant downregulation of SPTBN1 in plaque tissue compared to normal arteries or tissue adjacent to the plaque, respectively. To investigate the role of SPTBN1 in inflammation and vascular permeability in vivo, we assessed whether SPTBN1 expression is linked to intraplaque bleeding. C In the Maastricht Human Plaque Study cohort, sptbn1 expression showed an inverse correlation with the extent of intraplaque hemorrhage, here shown for one splicing variant (probe-1) (P = 0.0015; r2 = 0.2309). D In the independent Atheroexpress cohort, plaques of patients suffering from a cardiovascular event during follow-up had fourfold lower expression of SPTBN1 protein than event-free patients (E). In the same BIKE cohort, SPTBN1 mRNA expression was significantly lower in plaque of symptomatic vs asymptomatic patients. F The peptidomics dataset of the Maastricht Human Plaque Study was analyzed for peptides with significant correlation with module L; the corresponding protein list was strongly overrepresented in proteins of associated with hemorrhage (red) as well as proteins linked to ECM and endothelial cell adhesion (blue). Data are from n = 127 (A, E; BIKE), 36 (B; BIKE), 43 (C, F, Maastricht Human Plaque Study), and 28 (D; AtheroExpress) patient samples; error bars indicate SD; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005, ****p ≤ 0.001 |
|
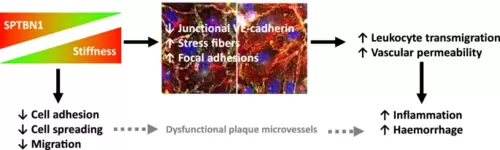
Schematic representation of the link between stiffness and SPTBN1 expression, and its effects in endothelial cells. SPTBN1 levels are reduced upon higher local tissue stiffness. Lower levels of SPTBN1 in turn lead to (1) reduced cell adhesion, spreading, and migration, which may lead to less developed plaque microvessels, and (2) altered endothelial junctions, more stress fibers, and more focal adhesions, which in turn enhance leukocyte transmigration and local vascular permeability. Together, this will lead to an increased local inflammatory response, as well as a higher occurrence of vascular leakage and intraplaque hemorrhage |