- Title
-
Hepatic extracellular ATP/adenosine dynamics in zebrafish models of alcoholic and metabolic steatotic liver disease
- Authors
- Tokumaru, T., Apolinario, M.E.C., Shimizu, N., Umeda, R., Honda, K., Shikano, K., Teranishi, H., Hikida, T., Hanada, T., Ohta, K., Li, Y., Murakami, K., Hanada, R.
- Source
- Full text @ Sci. Rep.
|
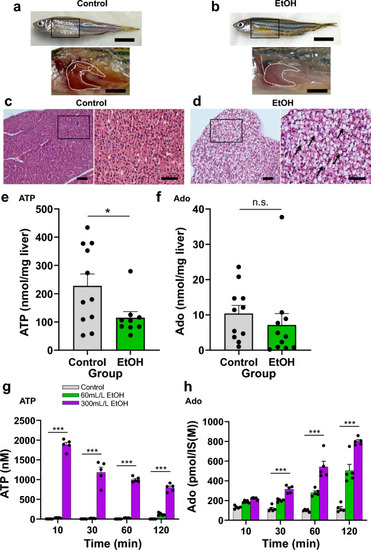
ATP and adenosine (Ado) content in the whole liver of adult zebrafish and HepG2 culture supernatant after ethanol (EtOH) exposure. ( |
|
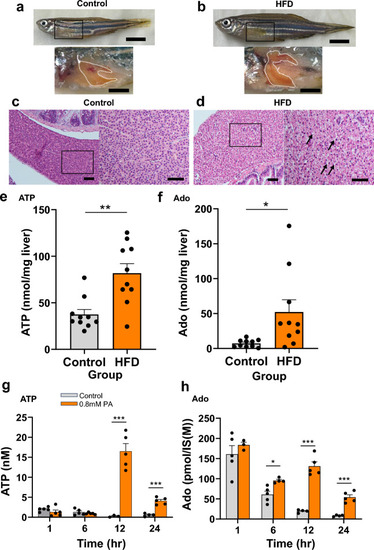
ATP and adenosine (Ado) content in the whole liver of adult zebrafish after high-fat diet (HFD) exposure and in HepG2 culture supernatant after palmitic acid (PA) treatment. ( |
|
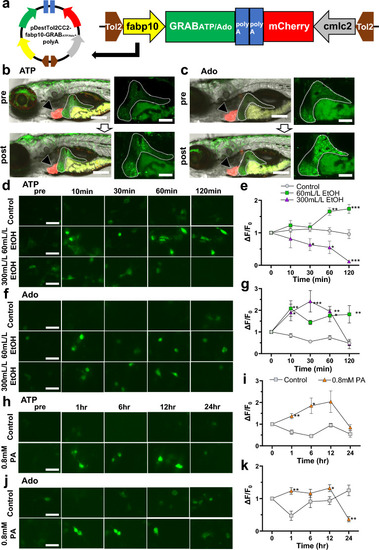
Establishment of hepatocyte-specific GRABATP and GRABAdo zebrafish models and confirmation of GRAB sensors in HepG2 cells. ( |
|
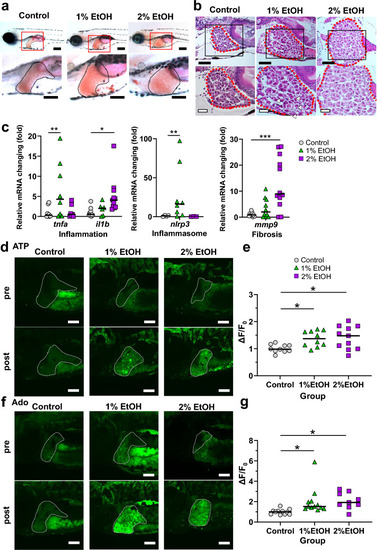
Ethanol treatment of zebrafish larvae increased the extrahepatic ATP and Ado levels. ( |
|
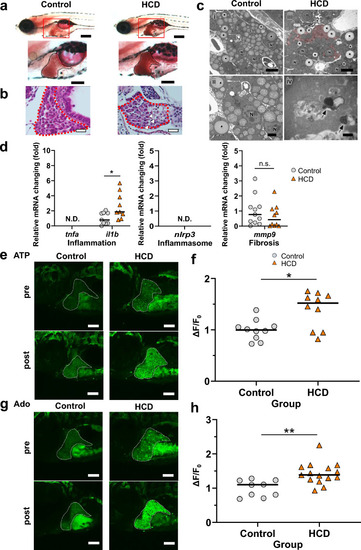
Cholesterol treatment of zebrafish larvae increased the extrahepatic ATP and Ado levels. ( |
|
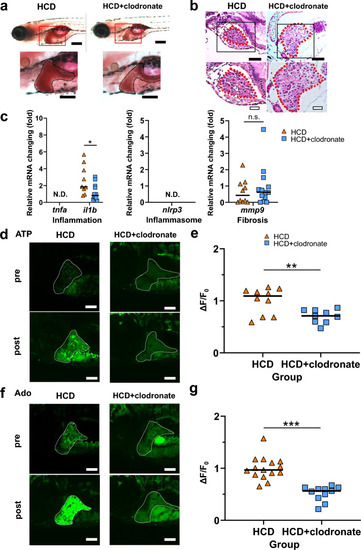
Clodronate improved the MASLD condition correlating with the reduction in extrahepatic ATP and adenosine (Ado) levels. ( |