- Title
-
The adaptor protein 2 (AP2) complex modulates habituation and behavioral selection across multiple pathways and time windows
- Authors
- Zúñiga Mouret, R., Greenbaum, J.P., Doll, H.M., Brody, E.M., Iacobucci, E.L., Roland, N.C., Simamora, R.C., Ruiz, I., Seymour, R., Ludwick, L., Krawitz, J.A., Groneberg, A.H., Marques, J.C., Laborde, A., Rajan, G., Del Bene, F., Orger, M.B., Jain, R.A.
- Source
- Full text @ iScience
|
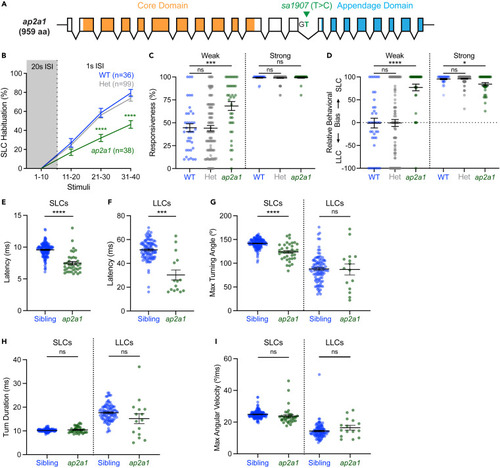
Diverse ap2s1 alleles disrupt acoustically evoked habituation learning, responsiveness, and behavior selection bias (A) Diagram of AP2 complex, annotated with the zebrafish genes encoding each subunit. The heterotetrametric complex is composed of AP2α (ap2a1, green), AP2β (ap2b1, dark gray), AP2σ (ap2s1, red), and AP2μ (ap2m1a, ap2m1b, light gray). (B) Diagram of ap2s1 transcript and alleles, regions contributing to AP2 target binding indicated in orange with mutant disruptions indicated in red. (C‒H) Acoustic SLC habituation of wild type sibling (WT, blue), heterozygote sibling (Het, gray) and ap2s1 mutants (red), presented with 10 intense (23.8 dB) stimuli at 20s ISI, followed by 30 more identical stimuli at 1s ISI. Individual habituation scores were normalized to their baseline SLC response rate at 20s ISI (gray shading), and ap2s1 mutants were compared to siblings with a 2-way repeated measures ANOVA (Genotype: p < 0.0001, Time: p < 0.0001, Individual: p < 0.0001), and significant pairwise comparisons (Dunnett’s test) vs. wild type are indicated, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Average SLC habituation scores across 300 identical stimuli at 1s ISI with hyperbolic fitted curves (F–H). (I‒K) Average responsiveness of individual larvae to 10 low intensity (−0.6 dB) acoustic stimuli presented at 20 s ISI, compared with two-tailed Mann-Whitney test with Bonferroni correction for multiple comparisons. (L‒N) Relative behavioral bias of individual larvae to 10 low intensity (−0.6 dB) acoustic stimuli presented at 20 s ISI, compared with two-tailed Mann-Whitney test with Bonferroni correction for multiple comparisons. Mutants (red) and heterozygotes (gray) were always compared to their corresponding wild type (blue) siblings, using ap2s1p172 (C, F, I, L), ap2s1hv1 (D, G, J, M), and ap2s1p199 (E, H, K, N) alleles. Data are represented as mean ± SEM in all panels and n indicates individual larvae. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. |
|
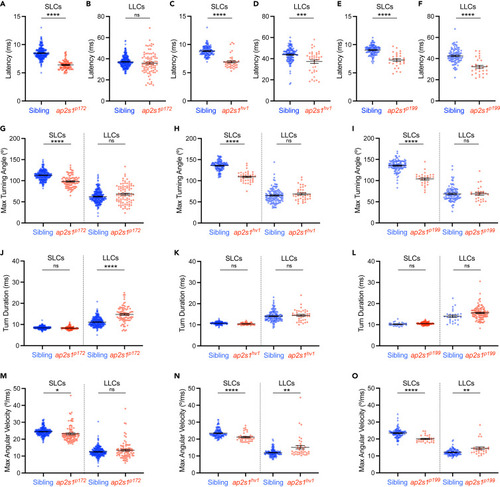
ap2s1 modulates distinct kinematic features of acoustically evoked SLC and LLC escape responses Average movement parameters of acoustically evoked behavioral responses of sibling (blue) and ap2s1 mutant (red) larvae across 40 strong (23.8 dB) acoustic stimuli, separated by SLC (left) and LLC (right) responses. (A‒F) Average acoustic response latencies for SLC (A,C,E) and LLC (B,D,F) behavior of ap2s1 mutants and their siblings. (G‒I) Average maximum turning angle of the initial C-bend of ap2s1 mutant and sibling responses. (J‒L) Average duration of the initial C-bend of ap2s1 mutant and sibling responses. (M‒O) Average maximal angular velocity of SLC and LLC responses of ap2s1 mutants and siblings. The ap2s1p172 allele was used in panels A, B, G, J, M (n = 260 siblings, n = 88 mutants), ap2s1hv1 was used in panels C, D, H, K, N (n = 123 siblings, n = 40 mutants), ap2s1p199 was used in panels E, F, I, L, O (n = 100 siblings, n = 28 mutants). Data are represented as mean ± SEM in all panels. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, two-tailed t-test with Welch’s correction. |
|
The AP2α subunit regulates acoustically evoked escape behavior (A) Diagram of ap2a1 transcript and disrupted splice donor site of ap2a1sa1907 (green). Core domain represented in orange and flexible appendage domain represented in blue. (B‒D) Acoustically evoked response modulation of wild types (blue, n = 36), heterozygotes (gray, n = 99) and ap2a1sa1907 mutants (green, n = 38). Average SLC habituation scores across 30 stimuli at 1s ISI (B), normalized to each individual’s baseline SLC response levels to 10 strong (23.8 dB) stimuli at 20s ISI. ap2a1 sa1907 mutants were compared to siblings with a 2-way repeated measures ANOVA (Genotype: p < 0.0001, Time: p < 0.0001, Individual: p < 0.0001), and significant pairwise comparisons (Dunnett’s test) vs. wild type are indicated, ∗∗∗∗p < 0.0001. Average responsiveness (C) and relative behavioral bias (D) of individuals to 10 weak (−6.7 dB) and 10 strong (23.8 dB) stimuli at 20 s ISI. ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, two-tailed Mann-Whitney test with Bonferroni correction for multiple comparisons. (E‒I) Average kinematic parameters of individual sibling (blue, n = 142) and ap2a1sa1907 mutant (green, n = 37) larvae in response to 40 strong (23.8 dB) acoustic stimuli, separated by SLC (left) and LLC (right) responses. Parameters measured were the initial response latency (E-F), maximum turning angle of the initial C-bend (G), initial C-bend duration (H), and maximum angular velocity of the initial C-bend (I). ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, two-tailed t-tests with Welch’s correction. Data are represented as mean ± SEM in all panels. |
|
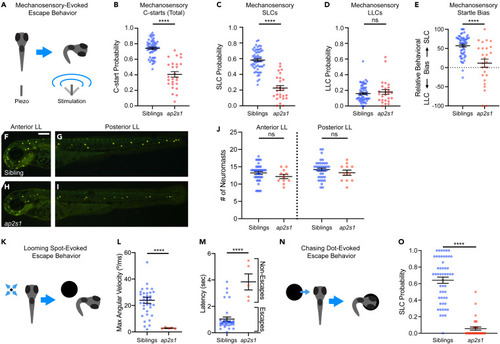
ap2s1 differentially regulates SLC and LLC escape behavior across varied stimulus modalities (A and B) C-start response probability of ap2s1p172 mutants (red, n = 26) to vibrating piezo stimuli compared to non-mutant siblings (blue, n = 56). ∗∗∗∗p < 0.0001, two-tailed t-test with Welch’s correction. (C‒E) Within the lateral line dependent mechanosensory-induced C-start responses, the probabilities of SLC responses (C) and LLC responses (D) for siblings (blue) and ap2s1p172 mutants (red), ∗∗∗∗p < 0.0001, ns = not significant, two-tailed t-test with Welch’s correction. Relative behavioral bias of lateral line dependent mechanosensory-evoked escape behaviors (E) for siblings and ap2s1p172 mutants, ∗∗∗∗p < 0.0001, two-tailed Mann-Whitney test. (F‒J) Distribution of sensory neuromasts of the anterior lateral line (F, H) and posterior lateral line (G, I) labeled with FM1-43X in 3 dpf ap2s1p172 mutants (red, n = 11) compared to non-mutant siblings (blue, n = 38), ns = not significant, two-tailed Mann-Whitney test. Scale bar is 200μm. (K‒M) Maximum angular velocity (L) and response latency (M) of individual behavioral responses of siblings (blue, n = 5) and ap2s1p172 mutants (red, n = 5) to looming visual stimuli. ∗∗∗∗p < 0.0001, two-tailed Student’s t test with Welch’s correction. (N‒O) Average SLC frequency of non-mutant siblings (blue, n = 53) and ap2s1p172 mutants (red, n = 33) in response to visual “chasing dot stimuli.” ∗∗∗∗p < 0.0001, two-tailed Mann-Whitney test. Data are represented as mean ± SEM in all panels. |
|
ap2s1 modulates visually evoked behavior selection and habituation learning (A) Total responsiveness (A) to 6 dark flash (DF) stimuli presented at 150 s ISI by sibling (blue, n = 234) and ap2s1p199 mutant larvae (red, n = 79), two-tailed Mann-Whitney test. (B) Response latency of large angle turns (>95°) by siblings (blue) and ap2s1p199 mutants (red) following DF stimuli in A, two-tailed Mann-Whitney test, ∗∗∗∗p < 0.0001. (C) Relative frequencies of 13 behavioral bout types in response to DFs between siblings (blue) and ap2s1p199 mutants (red). Forward swims and turning bouts are placed roughly in order of increasing vigor, as classified by Marques et al.,53 including Approach Swim (AS), Slow Swim 1 (Slow 1), Slow Swim 2 (Slow 2), Short Capture Swim (SCS), Long Capture Swim (LCS), Burst Swim (BS), J-turn (J-turn), High Angle Turn (HAT), Routine Turn (RT), Spot Avoidance Turn (SAT), O-Bend (O-Bend), Long Latency C-start (LLC), and Short Latency C-start (SLC). (D‒G) Absolute probabilities of DF response behaviors by siblings (blue) and ap2s1p199 mutants (red), focusing on SATs (D, Mann-Whitney U = 7842, two-tailed Mann-Whitney test, ∗∗∗p = 0.0001), LLCs (E, Mann-Whitney U = 10110, two-tailed Mann-Whitney test, p = 0.367), RTs (F, Mann-Whitney U = 6565, two-tailed Mann-Whitney test, ∗∗∗∗p < 0.0001), and O-bends (G, Mann-Whitney U = 570.5, two-tailed Mann-Whitney test, p = 0.921). See also Figure S1 for details. (H) Habituation of large angle turn responses of siblings (blue, n = 214) and ap2s1p199 mutants (red, n = 69) to eight blocks of six identical DF stimuli, presented at 150s ISI for the first block and 15s ISI for subsequent blocks. Habituation assessed by two-way repeated measures ANOVA (Genotype ∗∗p = 0.0043, Time ∗∗∗∗p < 0.0001, Individual ∗∗∗∗p < 0.0001). Data are represented as mean ± SEM in all panels. |
|
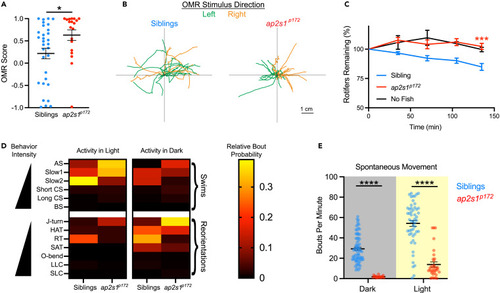
ap2s1 regulates behavioral performance and choice in goal-directed and spontaneous contexts (A) OMR score of siblings (blue, n = 16) and ap2s1p172 mutants (red, n = 11) to moving optomotor visual stimuli, where positive scores indicate overall movement in the same direction as the visual stimuli. ∗p < 0.05, two-tailed Mann-Whitney test. (B) Relative fish movement trajectories following the onset of each leftward (green) or rightward (orange) optomotor stimulus, depicted starting from the black cross at the center of each graph. Presented paths of siblings (left graph) and mutants (right graph) were truncated upon exiting a square region of interest during the trial (see STAR Methods). (C) Relative consumption of rotifers by non-mutant siblings (blue, n = 23), ap2s1p172 mutants (red, n = 11), and control arenas with no fish (black, n = 3) over a 135-min time period (∗∗∗p ≤ 0.001, two-tailed t-test with Welch’s correction, at conclusion of assay). Rotifer counts for each individual were normalized to 100% at time 0. (D and E) Sibling and ap2s1p172 mutant spontaneous behavior in the light (n = 52 siblings, n = 33 mutants) and the dark (n = 72 siblings, n = 18 mutants). ∗∗∗∗p < 0.0001, two-tailed Mann-Whitney test. See also Figure S2 for details. Data are presented as mean ± SEM in all panels. |
|
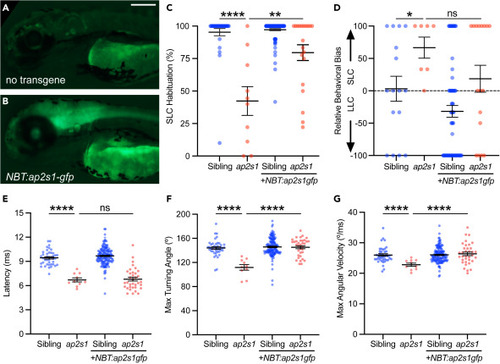
Neuronal expression of ap2s1 is sufficient for acoustic habituation and response kinematics (A and B) 3 dpf non-transgenic larva (A) and larva carrying Tg(NBT:ap2s1-gfp) (B) expressing green fluorescence in the brain and spinal cord. Scale bar is 200μm. (C) Acoustic habituation percentage for sibling (blue) and ap2s1p172 (red) larvae in the final 10 of 40 intense (23.8 dB) stimuli at 1s ISI (stimuli 31–40), with or without Tg(NBT:ap2s1-gfp) as indicated. ∗∗p < 0.01, ∗∗∗∗p < 0.0001, Bonferroni corrected one-tailed Mann-Whitney test. (D) Behavior selection bias of sibling (blue) and ap2s1p172 (red) larvae in response to weak stimuli (−8.2 dB). ∗p < 0.05, ns = not significant, Bonferroni corrected one-tailed Mann-Whitney test. (E‒G) Average kinematic parameters of SLC startle responses for sibling (blue) and ap2s1p172 (red) larvae: latency (E), maximal turning angle (F), maximal angular velocity (G). ∗∗∗∗p < 0.0001, ns = not significant, one-tailed t-test with Bonferroni correction. Data are represented as mean ± SEM in all panels. EXPRESSION / LABELING:
|
|
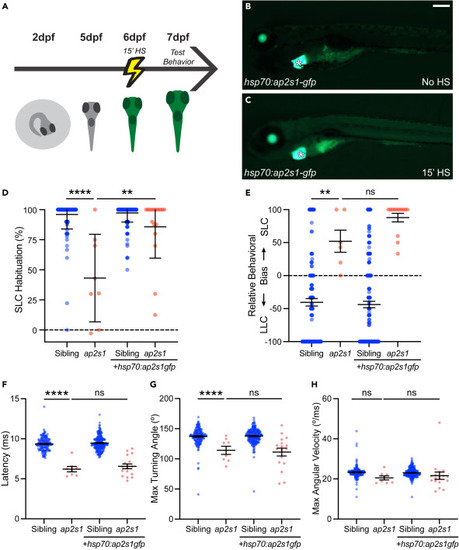
Acute expression of ap2s1 is sufficient for acoustic habituation (A) Timeline for acute induction of ap2s1-gfp expression in larvae. (B and C) 6 dpf larva carrying Tg(hsp70:ap2s1-gfp) without induction (B) and 5 h after a single 15-min 38°C heat shock treatment (C). The transgene is marked with an independent GFP marker in the heart (asterisk). Scale bar is 200μm. (D) Acoustic habituation percentage for sibling (blue) and ap2s1p172 (red) larvae in the final 10 of 40 intense (23.8 dB) stimuli at 1s ISI (stimuli 31–40), with or without Tg(hsp70:ap2s1-gfp) as indicated. All larvae were treated identically with a heat shock. ∗∗∗∗p < 0.0001, ∗∗p < 0.01, Bonferroni corrected one-tailed Mann-Whitney test. (E) Behavior selection bias of sibling (blue) and ap2s1p172 (red) larvae in response to weak stimuli (−8.2 dB). ∗∗p < 0.01, ns = not significant, Bonferroni corrected one-tailed Mann-Whitney test. (F‒H) Average kinematic parameters of SLC startle responses for sibling (blue) and ap2s1p172 (red) larvae: latency (F), maximal turning angle (G), and maximal angular velocity (H). ∗∗∗∗p < 0.0001, ns = not significant, Bonferroni-corrected one-tailed t-test with Welch’s correction. Data are represented as mean ± SEM in all panels. |