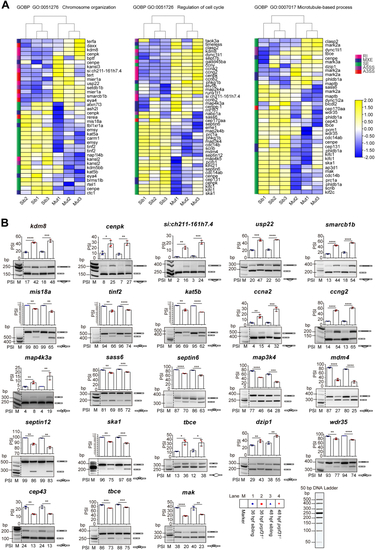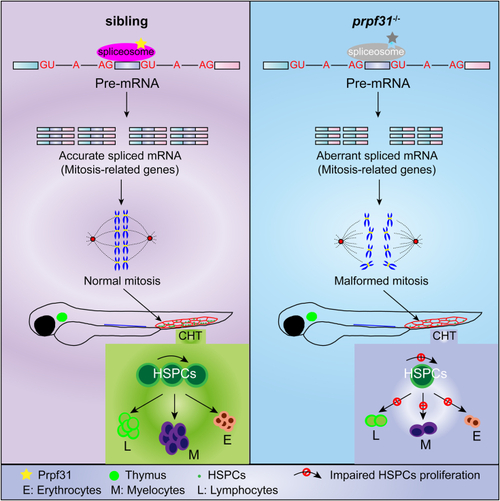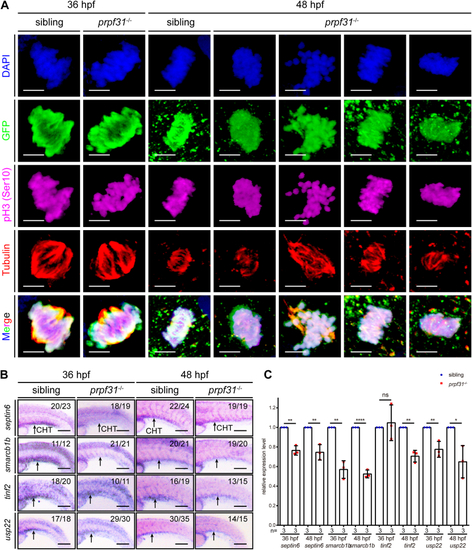
Aberrant alternative splicing of mitosis-related genes leads to mitotic malformations of HSPCs in the CHT of prpf31−/−zebrafish.A, quadruple staining of DAPI, cmyb:EGFP, pH3 (Ser10), and tubulin showed the mitotic status of HSPCs in the CHT of prpf31−/− zebrafish and siblings at 36 and 48 hpf. At least 25 mitotic HSPCs in more than six embryos were observed for each group. The scale bars represent 4 μm. B, expression of four aberrantly spliced mitosis-related genes (septin6, smarcb1b, tinf2, and usp22) in the CHT of siblings and prpf31−/− zebrafish at 36 and 48 hpf by WISH. Lateral views. Black arrows denote the CHT region. The number of embryos with similar gene expression patterns among all embryos examined were shown on the top right of each panel. The scale bars represent 100 μm. C, qRT-PCR analysis of four aberrantly spliced mitosis-related genes (septin6, smarcb1b, tinf2, and usp22) in siblings and prpf31−/− zebrafish at 36 and 48 hpf. Data were shown as mean ± SD of three independent experiments (n = 3); unpaired two-tailed t test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; ns, not significant. CHT, caudal hematopoietic tissue; hpf, hours postfertilization; HSPC, hematopoietic stem and progenitor cell; pH3, phospho-histone 3; qRT-PCR, quantitative real-time PCR; WISH, whole-mount in situ hybridization. DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein.
|