- Title
-
Convolamine, a tropane alkaloid extracted from Convolvulus plauricalis, is a potent sigma-1 receptor-positive modulator with cognitive and neuroprotective properties
- Authors
- Crouzier, L., Meunier, J., Carles, A., Morilleau, A., Vrigneau, C., Schmitt, M., Bourguignon, J.J., Delprat, B., Maurice, T.
- Source
- Full text @ Phytother. Res.
|
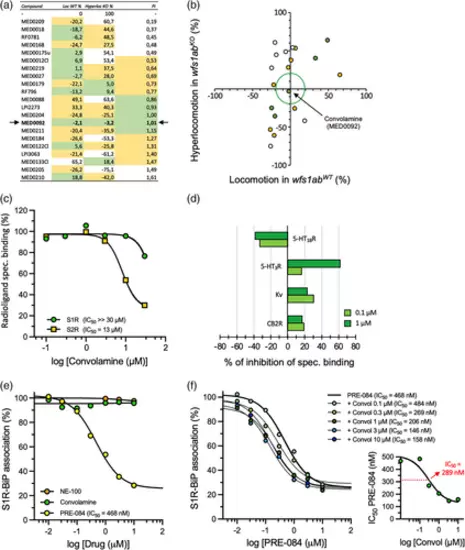
Identification of convolamine as an S1R positive modulator. (a) In vivo phenotypic drug screening in wfs1abKO zebrafish showing the effect of repositionable drugs on locomotor response in wfs1abWT controls (Loc WT %), on hyperlocomotor response in wfs1abKO zebrafish (Hyperloc KO %) and calculated PI. Convolamine is identified as MED0092. (b) Two-way graphic representation of the screening data in (a). Dots in green correspond to 0.8 < PI <1.2 and dots in yellow to 0.5 < PI <1.5. Only convolamine located within the green circle corresponding to effects on Loc WT % and Hyperloc KO % limited to 20%. (c) Dose–response effect of convolamine on inhibition of specific 3H-radioligand binding to S1R and S2R binding sites. Estimated IC50 are indicated in the graph. (d) The in vitro pharmacological screening on 47 receptors and binding sites showed only marked inhibition of convolamine on CB2 receptors, Kv channels, 5-HT3 receptor binding and stimulation of 5-HT1B receptors, at 0.1 or 1 μM. (e) S1R/BiP dissociation assay for PRE-084 (estimated IC50 of 468 nM), NE-100 and convolamine. GFP-S1R-oe CHO cells were incubated with the test compound for 30 min at 37°C before GFP immunoprecipitation. (f) Impact of fixed concentrations of convolamine (0.1–10 μM) on PRE-084-induced dissociation of S1R from BiP. Insert: the shift in IC50 values for PRE-084 was plotted versus Convolamine concentration and showed an IC50 for the positive modulatory effect of Convolamine at 289 nM |
|
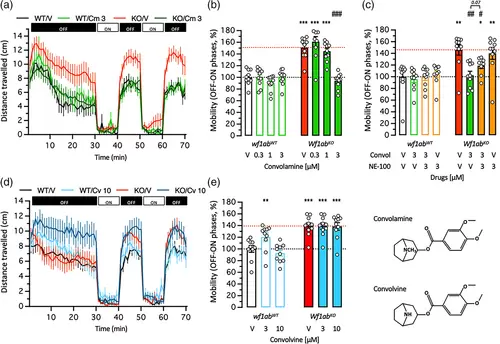
Effect of (a)–(c) Convolamine (Cm) and (d), (e) Convolvine (Cv) on the hyperlocomotor response of 5 dpf wfs1abWT and wfs1abKO mutant zebrafish larvae. (a), (d) VMR activity profiles. The activity is measured for 70 min, with 30 min of training in the dark (OFF), then 2 cycles of light/dark (ON/OFF) of 10 min each. (b), (c), (e) Analysis of the distance traveled by 5 dpf wfs1abWT and wfs1abKO larvae during the light/dark cycle in the VMR test as averaged differences between the OFF and ON phases. Data show mean ± SEM with n = 8–12 in (a), (b), n = 9–12 in (c), n = 9–20 in (d), (e). Two-way ANOVAs: F(1,72) = 320.3, p < 0.0001 for the genotype, F(3,72) = 43.24, p < 0.0001 for the treatment, F(3,72) = 47.43, p < 0.0001 for the interaction in (b); F(1,72) = 54.79, p < 0.0001 for the genotype, F(1,72) = 13.19, p < 0.0001 for the treatment, F(3,72) = 8.161, p < 0.0001 for the interaction in (c); F(1,54) = 152.9, p < 0.0001 for the genotype, F(1,54) = 10.13, p = 0.0002 for the treatment, F(3,54) = 6.636, p = 0.0027 for the interaction in (e). * p < 0.05, ** p < 0.01, *** p < 0.01 versus V-treated wfs1abWT group; # p < 0.05, ## p < 0.01, ### p < 0.0001 versus V-treated wfs1abKO group; Dunnett's test. |
|
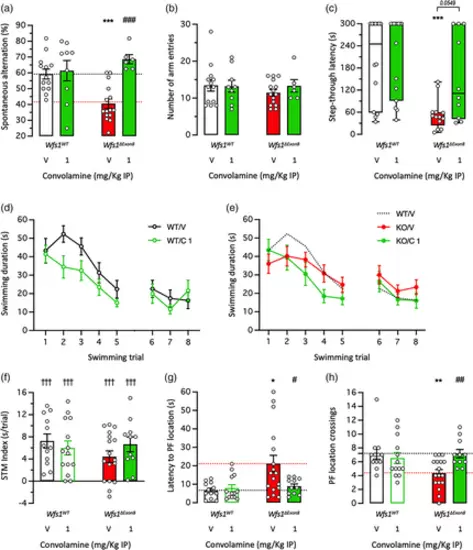
Neuroprotective effect of Convolamine on learning impairments in 3-month-old Wfs1∆Exon8 mice: (a), (b) spontaneous alternation in the Y-maze; (c) step-through passive avoidance; and (d)–(h) active avoidance in the rectangular water-maze. Animals received Convolamine (0.3 and 1 mg/kg IP) 20 min before the Y-maze session or training sessions for the passive and active avoidance tests. Retention was measured 24 h after training for passive avoidance and 72 h after training for active avoidance, without drug injection. (a) Spontaneous alternation percentage and (b) number of arm entries in the Y-maze test. (c) Step-through latency during the passive avoidance retention session. (d), (e) Active avoidance acquisition profiles in the rectangular water-maze, (f) short-term memory (STM) index, (g) latency to reach the platform location and (h) platform location crossings during the retention sesion. Bar graphs show mean ± SEM and individual data in (a), (b), (f)–(h) and box-and-wisker graphs show median and interquartile range and individual data in (c). The numbers of animals per groups were: n = 6–15 in (a), (b), n = 10–15 in (c), n = 11–17 in (d)–(h). Two-way ANOVAs: F(1,39) = 1.847, p > 0.05 for the genotype, F(1,39) = 12.87, p = 0.0009 for the treatment, F(1,39) = 9.362, p = 0.0040 for the interaction in (a); F(1,39) = 0.3975, p > 0.05 for the genotype, F(1,39) = 0.2478, p > 0.05 for the treatment, F(1,39) = 0.4856, p > 0.05 for the interaction in (b); F(1,49) = 1.220, p > 0.05 for the genotype, F(1,49) = 0.3765, p > 0.05 for the treatment, F(1,49) = 1.608, p > 0.05 for the interaction in (f); F(1,49) = 5.867, p = 0.0192 for the genotype, F(1,49) = 2.993, p > 0.05 for the treatment, F(1,49) = 4.442, p = 0.0402 for the interaction in (g); F(1,49) = 2.388, p > 0.05 for the genotype, F(1,49) = 2.388, p > 0.05 for the treatment, F(1,49) = 6.633, p = 0.0131 for the interaction in (h). Kruskal–Wallis ANOVA: H = 16.53, p = 0.0009 in (c). * p < 0.05, ** p < 0.01, *** p < 0.001 versus V-treated Wfs1WT group; # p < 0.05, ## p < 0.01, ### p < 0.001 versus V-treated Wfs1∆Exon8 group; ††† p < 0.001 versus“0” level; Dunnett's test in (a), (b), (g), (h), Dunn's test in (c), one-column t-test in (f). |
|
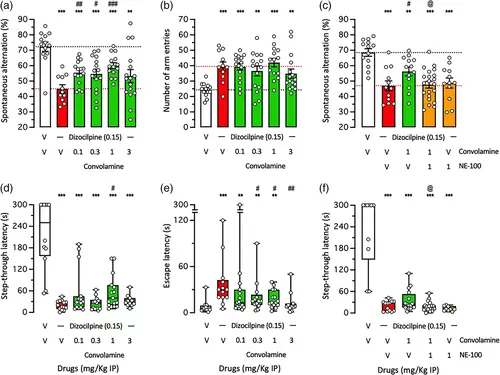
Anti-amnesic effect of Convolamine against Dizocilpine-induced learning impairments in mice: (a)–(c) spontaneous alternation test in the Y-maze and (d)–(f) step-through passive avoidance. Animals received Convolamine (0.1–3 mg/kg IP) 10 min before Dizocilpine (0.15 mg/kg IP), 20 min before the Y-maze session or passive avoidance training session. Retention was measured 24 h after training. (a), (c) alternation percentage and (b) number of arm entries in the Y-maze test. (d), (f) Step-through latency and (e) escape latency during the passive avoidance retention session. In (c), (f) NE-100 (1 mg/kg IP) was administered simultaneously as Convolamine (1 mg/kg IP), 10 min before Dizocilpine. Bar graphs show mean ± SEM and individual data in (a)–(c) and box-and-wisker graphs show median and interquartile range and individual data in (d)–(f). The number of animals per groups were: n = 11–16 in (a), (b), 12–21 in (c), 11–15 in (d), (e), and n = 10–18 in (F). ANOVA: F(5,79) = 8.085, p < 0.0001, in (a); F(5,79) = 5.234, p = 0.0003, in (b); F(4,66) = 10.73, p < 0.0001, in (c). Kruskal–Wallis ANOVA: H = 32.99, p < 0.0001, in (d); H = 21.1, p = 0.0008, in (e); H = 29.67, p < 0.0001, in (f). ** p < 0.01, *** p < 0.001 versus (V + V)-treated group; # p < 0.05, ## p < 0.01, ### p < 0.0001 versus (V + Dizocilpine)-treated group; @ p < 0.05 versus (Convolamine 1 + Dizocilpine)-treated group; Dunnett's test in (a)–(c), Dunn's test in (d)–(f). |
|
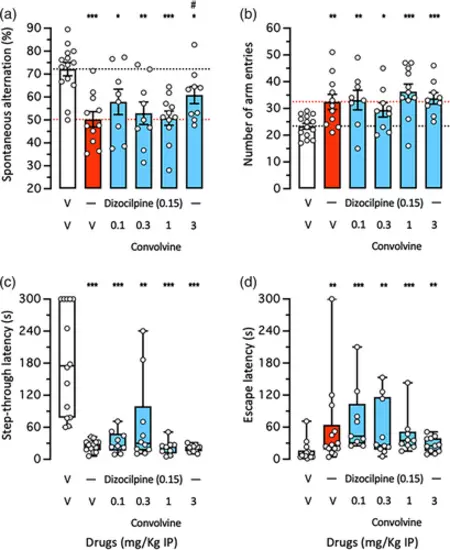
Anti-amnesic effect of Convolvine against Dizocilpine-induced learning impairments in mice: (a), (b) spontaneous alternation in the Y-maze and (c), (d) step-through passive avoidance. Animals received Convolvine (0.1–3 mg/kg IP) 10 min before Dizocilpine (0.15 mg/kg IP), 20 min before the Y-maze session or passive avoidance training session. Retention was measured 24 h after training. (a) Spontaneous alternation percentage and (b) number of arm entries in the Y-maze test. (c) Step-through latency and (d) escape latency during the passive avoidance retention session. Bar graphs show mean ± SEM and individual data in (a), (b) and box-and-wisker graphs show median and interquartile range and individual data in (c), (d). The number of animals per groups were: n = 9–14 in (a)–(d). ANOVA: F(5,56) = 5.880, p = 0.0002, in (a); F(5,56) = 4.002, p = 0.0036, in (b). Kruskal–Wallis ANOVA: H = 32.73, p < 0.0001, in (c); H = 20.39, p = 0.0011, in (d). * p < 0.05, ** p < 0.01, *** p < 0.001 versus (V + V)-treated group; # p < 0.05 versus (V + Dizocilpine)-treated group; Dunnett's test in (a)–(c), Dunn's test in (d)–(f). |
|
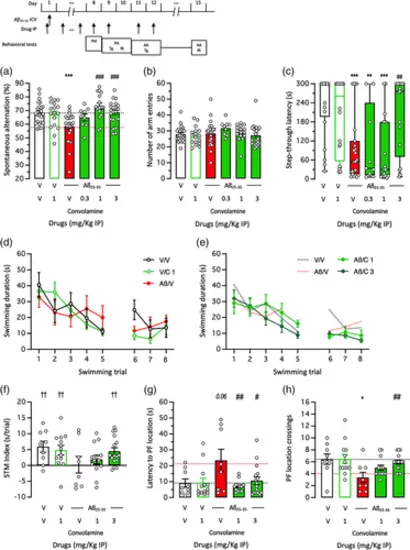
Neuroprotective effect of Convolamine against Aβ25-35-induced learning impairments in mice: (a), (b) spontaneous alternation in the Y-maze, (c) step-through passive avoidance, and (d)–(h) active avoidance in the rectangular water-maze. Animals received Aβ25-35 peptide (9 nmol ICV) on day 1 and Convolamine (0.3–3 mg/kg IP) o.d. from day 1 to day 12, as shown. Behavioral testing was performed between day 8 and day 15 with the drug being injected 20 min before the Y-maze session or the passive and active avoidance training sessions. Retention was measured 24 h after training for passive avoidance and 72 h after training for active avoidance, without drug injection. (a) Spontaneous alternation percentage and (b) number of arm entries in the Y-maze test. (c) Step-through latency during the passive avoidance retention session. (d), (e) Active avoidance acquisition profiles in the rectangular water-maze, (f) short-term memory (STM) index, (g) latency to reach the platform location and (h) platform location crossings during the retention sesion. Bar graphs show mean ± SEM and individual data in (a), (b), (f)–(h) and box-and-wisker graphs show median and interquartile range and individual data in (C). The numbers of animals per groups were: n = 9–24 in (a), (b), 9–23 in (c), 9–16 in (d)–(h). ANOVA: F(5,105) = 6.061, p < 0.0001, in (a); F(5,105) = 0.9373, p > 0.05, in (b); F(4,54) = 3.342, p = 0.0162, in (g); F(5,54) = 3.434, p = 0.0142, in (h). Kruskal–Wallis ANOVA: H = 26.75, p < 0.0001, in (c). * p < 0.05, ** p < 0.01, *** p < 0.001 versus (V + V)-treated group; # p < 0.05, ## p < 0.01, ### p < 0.001 versus (V + Aβ25-35)-treated group; †† p < 0.01 versus “0″ level; Dunnett's test in (a), (b), (g), (h), Dunn's test in (c), one-column t-test in (f). |
|
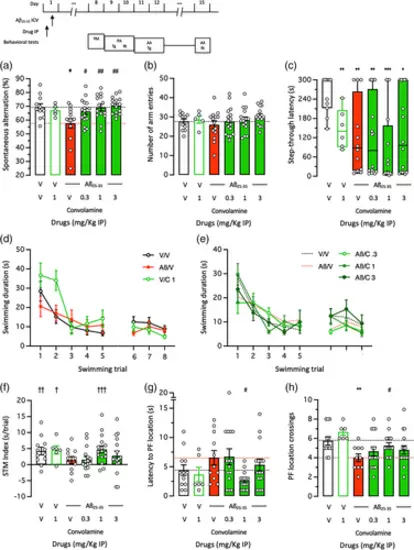
Neuroprotective effect of Convolamine against Aβ25-35-induced learning impairments in mice: (a), (b) spontaneous alternation in the Y-maze, (c) step-through passive avoidance, and (d)–(h) active avoidance in the rectangular water-maze. Animals received Convolamine (0.3–3 mg/kg IP) 20 min before Aβ25-35 peptide (9 nmol ICV) on day 1 and the Y-maze test was performed on day 8, the passive avoidance test on days 9–10 and the active avoidance test on days 11–12, as shown. Retention was measured 24 h after training for passive avoidance and 72 h after training for active avoidance, without drug injection. (a) Spontaneous alternation percentage and (b) number of arm entries in the Y-maze test. (c) Step-through latency during the passive avoidance retention session. (d), (e) Active avoidance acquisition profiles in the rectangular water-maze, (f) short-term memory (STM) index, (g) latency to reach the platform location and (h) platform location crossings during the retention sesion. Bar graphs show mean ± SEM and individual data in (a), (b), (f)–(h) and box-and-wisker graphs show median and interquartile range and individual data in (c). The numbers of animals per groups were: n = 5–16 in (a), (b), 6–16 in (c)–(h). ANOVA: F(5,69) = 3.587, p = 0.0068, in (a); F(5,69) = 0.8110, p > 0.05, in (b); F(5,68) = 2.465, p = 0.0412, in (g); F(5,68) = 3.206, p = 0.0117, in (h). Kruskal–Wallis ANOVA: H = 1.973, p > 0.05, in (c). * p < 0.05, ** p < 0.01, *** p < 0.001 versus (V + V)-treated group; # p < 0.05, ## p < 0.01 versus (V + Aβ25-35)-treated group; † p < 0.05, †† p < 0.01, ††† p < 0.001 versus “0″ level; Dunnett's test in (a), (b), (g), (h), Dunn's test in (c), one-column t-test in (f). |