- Title
-
Downregulation of Zebrafish Cytosolic Sialidase Neu3.2 Affects Skeletal Muscle Development
- Authors
- Zizioli, D., Codenotti, S., Benaglia, G., Manzoni, M., Massardi, E., Fanzani, A., Borsani, G., Monti, E.
- Source
- Full text @ Int. J. Mol. Sci.
|
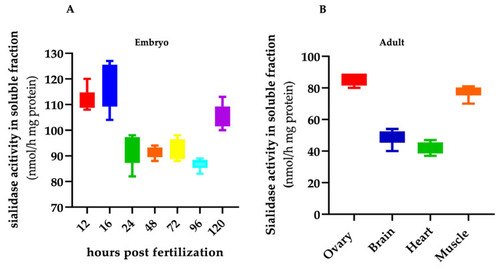
Soluble sialidase activity in zebrafish embryo and adult organs. Total extracts from wild-type embryos ( |
|
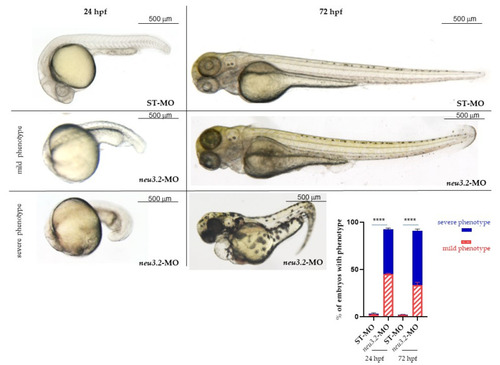
Effects of neu3.2 morpholino injection on zebrafish development. Representative images of the phenotype at 24 and 72 hpf obtained after the injection of neu3.2-MO. The injected embryos were compared with embryos injected with the standard morpholino (STD-MO). The embryos were injected with 1 pmol/embryo of |
|
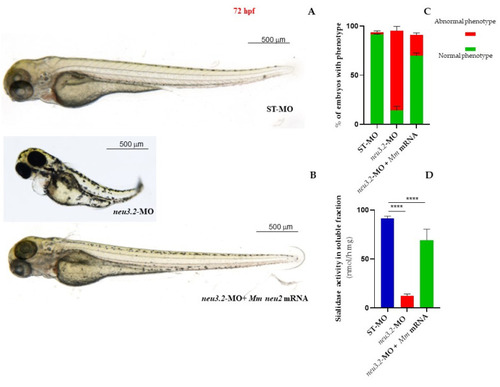
Rescue of the phenotype in |
|
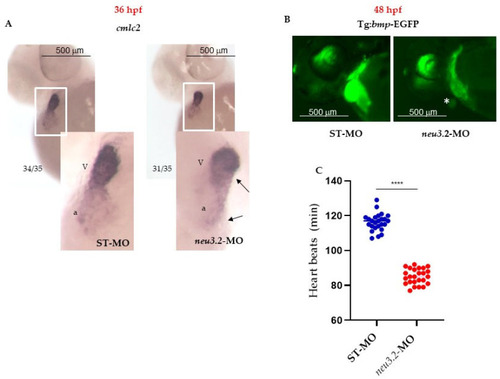
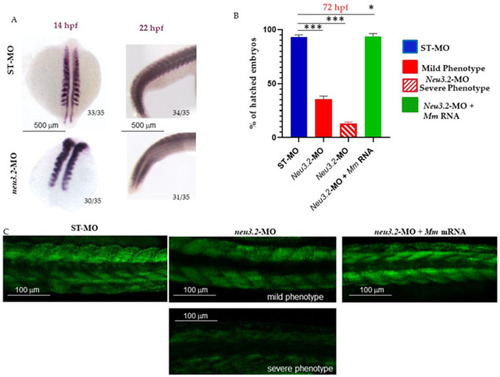
Downregulation of |
|
|
|
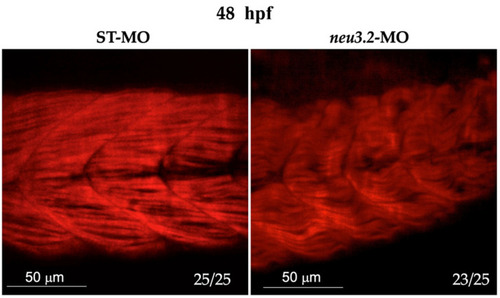
Knockdown of |
|
Expression level analysis of different markers involved in muscle development in neu3.2 morphants and embryos injected with the standard morpholino (STD-MO). Gene expression was normalized using |
|
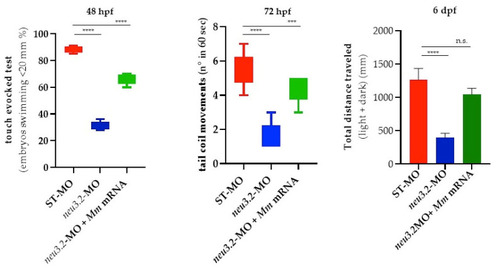
Locomotor analysis of neu3.2 morphants and murine Neu2-rescued embryos at different stages of development. Swimming performance of embryos at 48 hpf in the touch-evoked test. A significant progressive decrease in the percentage of |